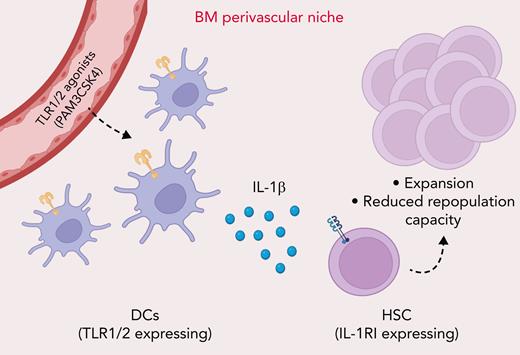
Bone marrow dendritic cells (BMDCs) are increasingly recognized as an important cellular component of the hematopoietic stem cell (HSC) perivascular niche. In this issue of Blood, Li et al1 report that BMDCs respond to Toll-like receptor 1/2 (TLR1/2) agonist stimulation by producing interleukin 1β (IL-1β), which is sensed by HSCs, leading to their expansion.
Mature hematopoietic cell production during both homeostasis and stress relies on a fine-tuned process of environmental sensing and intracellular signaling by HSCs within their microenvironment, also referred to as the “HSC niche.” Integration of cellular extrinsic signals such as inflammatory or pathogen-associated molecular pattern molecules (PAMPs) into cellular intrinsic molecular circuits determines whether HSCs remain quiescent or engage in multilineage differentiation and/or self-renewal divisions to generate the blood cells in demand, while at the same time maintaining a sufficient HSC pool size.2 HSCs can be activated via direct PAMP sensing by TLRs.3 In addition, PAMPs are detected by multiple BM cell types, leading to inflammatory cytokine production that ultimately determines HSC function.4 However, which cells, and possibly which specific perivascular niche cells are responsible for PAMP sensing and which inflammatory signals function as messengers to fine-tune HSC and hematopoietic stem and progenitor cell responses, is still not clear. In this issue of Blood, Li et al identify BMDCs as major cellular sensors of TLR1/2 agonists and show that this sensing results in increased BMDC IL-1β production, signaling HSCs to transiently expand their pool (see figure).
TLR1/2 agonist sensing by BMDCs results in increased production of the inflammatory cytokine IL-1β in the BM perivascular niche. Increased IL-1β levels are sensed by HSCs, leading to their pool expansion, biased myeloid differentiation, and reduced repopulation capacity.
TLR1/2 agonist sensing by BMDCs results in increased production of the inflammatory cytokine IL-1β in the BM perivascular niche. Increased IL-1β levels are sensed by HSCs, leading to their pool expansion, biased myeloid differentiation, and reduced repopulation capacity.
To identify the mouse BM myeloid phagocyte populations with the highest TLR expression levels, the authors preformed RNA-sequencing on DCs (CX3CR1high MHCIIhigh CD11chigh Gr-1– B220–), macrophages (CX3CR1– MHCIIhigh CD169+ Gr-1– B220–), and monocytes (CX3CR1high CD115+ Gr-1+ B220–) and observed that DCs exhibit the highest levels of Tlr1/2 and inflammatory Il1b expression. In vivo exposure to a TLR1/2 agonist (PAM3CSK4) resulted in an overall decrease of BM myeloid phagocytic populations, decreased osteoblast/mesenchymal stromal cell/sinusoidal endothelial cell numbers, and increased mobilization and expansion of phenotypically defined HSCs due to higher HSC cycling activity. Importantly, using a “conventional” DC-specific deletion of the TLR pathway member Myd88 (Zbtb46-Cre, Myd88f/f mice), Li et al show that only HSC expansion is directly dependent on DC-specific TLR sensing. This is a major finding, with 2 key implications: (1) it excludes major cell-autonomous effects of the TLR1/2 agonist in BM HSC proliferation, challenging the notion that HSCs are, at least partially, direct sensors of this specific type of PAMP5; and (2) it links HSC expansion downstream of TLR1/2 to a perivascular niche cell type, which had previously been shown for the sensing of microbiota-derived circulating bacterial DNA by BM CX3CR1+MHCIIhi mononuclear cells during steady-state hematopoiesis.4 Importantly, whether DCs can also sense steady-state levels of microbiota-derived TLR1/2 agonists and not just hyper-physiological doses of PAM3CSK4 remains to be determined.
To address the mechanism by which DCs contribute to PAM3CSK4-induced HSC expansion, Li et al performed RNA-sequencing on sorted BMDCs 24 hours after treatment with PAM3CSK4. The authors identified Il1b upregulation as a potential candidate, thereby linking DCs to HSC expansion. Indeed, IL-1β chronic exposure was shown to cause HSCs and multipotent progenitor cell expansion and enhanced HSC myeloid differentiation, while impairing HSC reconstitution capacity.6 To test if IL-1β was the molecular driver of HSC proliferation, the authors exposed IL-1R1−/− mice to PAM3CSK4 and observed reduced HSC expansion compared with wild-type (WT) animals, strongly suggesting that TLR1/2-induced HSC and progenitor cell expansion is dependent on IL-1 signaling. Of note, there was still a minor but significant expansion of LSK (Lin– Sca-1+ C-Kit+) and multipotent progenitor 3 (MPP3) in IL-1R1−/− mice after PAM3CSK4 exposure, suggesting that additional inflammatory mediators may be involved. Moreover, as expected from chronic IL-1β exposure studies, while PAM3CSK4-treated WT HSCs lost repopulation capacity, their IL-1R1−/− counterparts were able to maintain repopulation capacity upon secondary transplantation.
Finally, the authors cultured sorted murine WT HSCs with conditioned media from PAM3CSK4-stimulated BMDCs and observed that IL-1β, present in the conditioned media, was indeed able to increase the Lin– C-Kit+ population cell number. As further confirmation of this finding, exposure of human DCs, but not monocytes, to PAM3CSK4 resulted in increased IL-1β levels. This finding suggests that PAM3CSK4 treatment induces an expansion of hematopoietic progenitors, at least in part, by increasing IL-1β expression in BMDCs. Overall, this study establishes BMDC-mediated IL-1β production downstream of TLR1/2 activation as a key molecular bridge between PAMP-sensing and HSC responses. These findings align with and extend other recent data, in which we observed an age-dependent increase in circulating microbiota-derived TLR4/8 agonists. These agonists are sensed by BM inflammatory monocytes and granulocytes, causing increased IL-1 production that ultimately leads to HSC pool expansion and an increased myeloid differentiation bias.7 Integration of these findings highlights IL-1 as a major inflammatory cytokine induced downstream of TLR signaling within different BM myeloid cell types influencing HSC proliferation and myelopoiesis.
Moreover, the findings by Li et al implicate TLR1/2 and IL-1 signaling in the development of hematologic dysfunction. Indeed, the authors show increased TLR1 and IL-1β expression in BMDCs, CD14+ monocytes, and CD16+ monocytes in a small number of human myelodysplastic syndrome (MDS) samples. Although this finding needs validation in larger cohorts, it aligns with previous reports on increased TLR1/2 signaling in MDS hematopoietic progenitors.8 Moreover, altered IL-1 signaling has also been implicated in MDS pathology and development of acute myeloid leukemia (AML). Expression of IL-1 accessory protein (eg, IL1RAP, a IL-1R1 coreceptor) is increased in high-risk MDS and AML blasts and associates with poor overall survival in patients with AML.9 It is therefore tempting to speculate that in pre-leukemic states such as MDS (and potentially also in clonal hematopoiesis), enhanced TLR1/2 sensing can lead to higher IL-1 production; this might favor the selection of mutant HSCs over WT counterpart cells, leading to expansion of inflammation-adapted mutant clones and further accelerating progression to full leukemia. The findings by Li et al further highlight the need to understand whether leukemia-founding mutations might not only be present in BMDCs but also might alter and possibly enhance their inflammatory responses, as observed for DNMT3a and TET2 mutant myeloid cells.10
Overall, the findings by Li et al identify BMDCs as active players of the HSC niche with a critical role in PAMP sensing and IL-1–dependent regulation of HSC expansion. Future studies will need to unravel the precise contribution of multiple PAMP-sensing cell types as well as the molecular pathways operating in various infectious/inflammatory contexts and their overall implications for host defense responses, hematologic aging, and disease development.
Conflict-of-interest disclosure: The authors declare no competing financial interests.