TO THE EDITOR:
Patients with myelodysplastic syndromes (MDS) often develop anemia,1 which is associated with fatigue, reduced quality of life, and increased hospitalization and mortality.2-5 Anemia is often managed with red blood cell (RBC) transfusions1; ∼50% of patients with lower-risk MDS (LR-MDS) require RBC transfusions within 2 years of diagnosis.6 Chronic RBC transfusions are associated with decreased quality of life,5,7,8 increased risk of iron overload, and reduced survival9,10 and come with significant health system costs and strain to the limited space in clinic and infusion areas, which intensified during the coronavirus disease 2019 pandemic.11,12
Based on the phase 3, double-blind, MEDALIST study (NCT02631070), luspatercept was approved in the United States and Europe for the treatment of anemia after failure of an erythropoiesis-stimulating agent (ESA; or in patients who are unlikely to respond to ESAs), when ≥2 RBC units are required over 8 weeks in adult patients with revised International Prognostic Scoring System (IPSS-R) very low- to intermediate-risk MDS with ring-sideroblasts (RS), or with MDS/myeloproliferative neoplasm with RS and thrombocytosis.13,14 Here, we report longer-term results from the MEDALIST trial with double the follow-up time of the primary analysis.14
Eligible patients (≥18 years) had anemia owing to LR-MDS-RS (defined as IPSS-R very low, low, or intermediate risk15,16); received regular RBC transfusions (≥2 units per 8 weeks during the 16 weeks before randomization); were refractory to, intolerant of, or unlikely to respond to (ie, serum erythropoietin >200 U/L) ESAs; and had not received disease-modifying agents (supplemental Figure 1, available on the Blood website). In the primary analysis, baseline transfusion burden was categorized as receiving <4 RBC units per 8 weeks, 4 to <6 RBC units per 8 weeks, or ≥6 RBC units per 8 weeks14; therefore, in the current post hoc analysis, patients receiving ≥6 RBC units within 8 weeks prior to randomization were classified as having high transfusion burden (HTB), whereas those receiving 2 to <6 RBC units were classified as having low transfusion burden (LTB).
Figure 1A shows baseline characteristics of the 229 randomized patients (luspatercept, N = 153; placebo, N = 76). As of July 1, 2019, 41 patients (26.8%) were still receiving luspatercept after >2 years, with some still on treatment after 3 years, but all patients receiving placebo had discontinued treatment. Median follow-up times for the current analysis were 26.4 and 26.1 months for luspatercept and placebo, respectively, compared with 13.9 months and 14.3 months for the primary analysis.
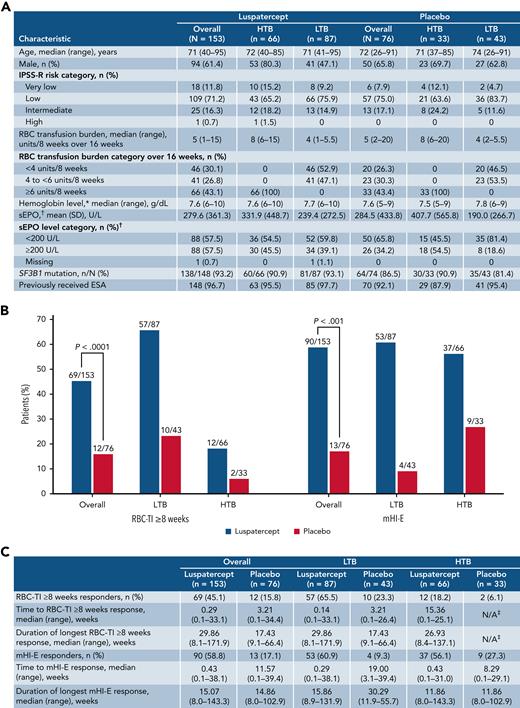
Patient baseline characteristics, RBC-TI ≥8 weeks and mHI-E response. Baseline characteristics of patients in the MEDALIST trial by transfusion burden (A). Rates of RBC-TI for ≥8 weeks and mHI-E response during weeks 1 to 48, overall and by transfusion burden (B). Time to first response and duration of RBC-TI ≥8 weeks and mHI-E response (C). mHI-E response was defined according to IWG 2006 criteria17 as a mean hemoglobin increase ≥1.5 g/dL among patients with a baseline RBC transfusion burden <4 units per 8 weeks or a reduction of ≥4 RBC units among patients with baseline RBC transfusion burden ≥4 units per 8 weeks, sustained over a consecutive 56-day period. ∗Last value measured on or before the date and time of the first dose of luspatercept per placebo. †Highest value within 35 days before the first dose of luspatercept per placebo. ‡Only 2 patients with HTB who received placebo achieved RBC-TI; therefore, a median duration could not be reliably estimated. IWG, International Working Group; mHI-E, modified hematologic improvement-erythroid; SD, standard deviation; sEPO, serum erythropoietin; SF3B1, splicing factor 3b subunit 1.
Patient baseline characteristics, RBC-TI ≥8 weeks and mHI-E response. Baseline characteristics of patients in the MEDALIST trial by transfusion burden (A). Rates of RBC-TI for ≥8 weeks and mHI-E response during weeks 1 to 48, overall and by transfusion burden (B). Time to first response and duration of RBC-TI ≥8 weeks and mHI-E response (C). mHI-E response was defined according to IWG 2006 criteria17 as a mean hemoglobin increase ≥1.5 g/dL among patients with a baseline RBC transfusion burden <4 units per 8 weeks or a reduction of ≥4 RBC units among patients with baseline RBC transfusion burden ≥4 units per 8 weeks, sustained over a consecutive 56-day period. ∗Last value measured on or before the date and time of the first dose of luspatercept per placebo. †Highest value within 35 days before the first dose of luspatercept per placebo. ‡Only 2 patients with HTB who received placebo achieved RBC-TI; therefore, a median duration could not be reliably estimated. IWG, International Working Group; mHI-E, modified hematologic improvement-erythroid; SD, standard deviation; sEPO, serum erythropoietin; SF3B1, splicing factor 3b subunit 1.
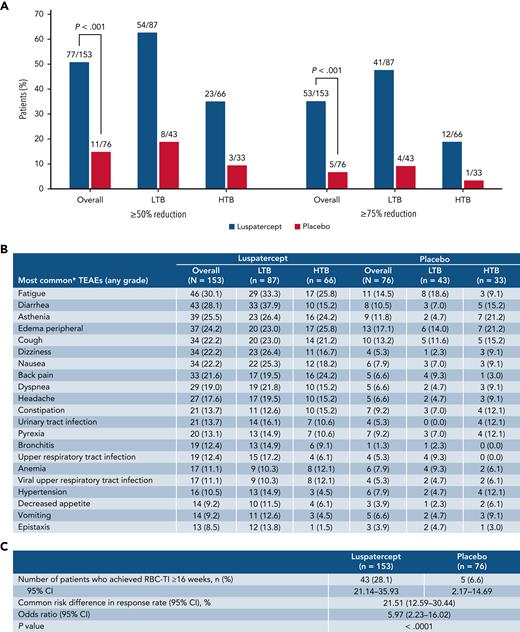
Approximately 3 times as many patients receiving luspatercept vs placebo achieved RBC-transfusion independence (RBC-TI) for ≥8 weeks during weeks 1 to 48 (69/153 [45.1%] vs 12/76 [15.8%]; P < .0001; Figure 1B). Forty-six of 73 patients who achieved RBC-TI ≥8 weeks at any time during the entire treatment period were TI at 1 year (supplemental Figure 1). Furthermore, during weeks 1 to 48, RBC-TI ≥16 weeks was achieved by 43/153 (28.1%) and 5/76 (6.6%) patients in the luspatercept and placebo arms, respectively (P < .0001; Figure 2C).
Transfusion burden reduction, TEAEs, and RBC-TI ≥16 weeks. Rates of ≥50% and ≥75% reduction in RBC transfusion burden from baseline over ≥24 weeks during the entire treatment phase, overall, and by transfusion burden (A). Summary of TEAEs during the entire treatment period (B). Rates of RBC-TI ≥16 weeks during weeks 1 to 48. Data are n (%). Transfusion events and TEAEs are reported during weeks 1 to 48. ∗Those occurring in ≥10% in any group. CI, confidence interval; TEAE, treatment emergent.
Transfusion burden reduction, TEAEs, and RBC-TI ≥16 weeks. Rates of ≥50% and ≥75% reduction in RBC transfusion burden from baseline over ≥24 weeks during the entire treatment phase, overall, and by transfusion burden (A). Summary of TEAEs during the entire treatment period (B). Rates of RBC-TI ≥16 weeks during weeks 1 to 48. Data are n (%). Transfusion events and TEAEs are reported during weeks 1 to 48. ∗Those occurring in ≥10% in any group. CI, confidence interval; TEAE, treatment emergent.
During the primary analysis, 37.9% of patients receiving luspatercept achieved RBC-TI for ≥8 weeks during weeks 1 to 24 vs 13.2% for placebo (P < .001).14 The increase from 37.9% to 45.1% among patients receiving luspatercept suggests additional patients would achieve RBC-TI beyond the primary 24-week follow-up period. A similar effect was observed among patients with HTB (12/66 [18.2%] vs 2/33 [6.1%]) and LTB (57/87 [65.5%] vs 10/43 [23.3%]) (Figure 1B). Lower rates of achievement of RBC-TI ≥8 weeks by HTB patients vs LTB patients reflect the difficulty for patients with HTB LR-MDS to achieve TI with any therapy.
From weeks 1 to 48, the median (range) hemoglobin levels in HTB patients during the longest period of RBC-TI ≥8 weeks receiving luspatercept and placebo were 98.9 g/dL (85.8-107.5) and 95.3 g/dL (94.8-95.9), respectively, and in LTB patients, they were 92.6 g/dL (74.2-113.4) and 89.8 g/dL (82.9-96.8), respectively.
Overall, the median (range) duration of RBC-TI ≥8 weeks response was 29.9 weeks (8.1-171.9) for patients receiving luspatercept and 17.4 weeks (9.1-66.4) for placebo. The median duration of RBC-TI for the 12/66 (18.2%) HTB patients receiving luspatercept who achieved RBC-TI ≥8 weeks was 26.9 weeks (range, 8.4-137.1). Given only 2/33 (6.1%) HTB patients receiving placebo achieved RBC-TI ≥8 weeks, a median duration of response could not be reliably estimated. For LTB patients, median (range) duration of RBC-TI was 29.9 weeks (8.1-171.9) and 17.4 weeks (9.1-66.4) for patients receiving luspatercept and placebo, respectively (Figure 1C).
Overall, during weeks 1 to 48, 31/153 (20.3%) patients receiving luspatercept achieved >1 period of RBC-TI ≥8 weeks response, including 29/87 (33.3%) LTB and 2/66 (3.0%) HTB patients, compared with 3/76 (3.9%) patients receiving placebo, whom were LTB patients.
During weeks 1 to 48, significantly more patients receiving luspatercept achieved a modified hematologic improvement-erythroid (mHI-E) response (per IWG 2006 criteria17) vs placebo (58.8% [95% confidence interval (CI), 50.6-66.7] vs 17.1% [95% CI, 9.4-27.5]; P < .001). Rates of mHI-E achievement increased from 52.9% of patients during weeks 1 to 24 in the primary analysis14 to 58.8% in the current analysis. Rates of mHI-E response were comparable for luspatercept between HTB patients and LTB patients, 56.1% (95% CI, 43.3-68.3) and 60.9% (95% CI, 49.9-71.2), respectively (Figure 1C), as was time to mHI-E (HTB 0.43 weeks vs LTB 0.29 weeks), while duration of mHI-E was slightly longer in LTB patients (HTB 11.9 weeks vs LTB 15.9 weeks).
During the entire treatment phase, a significantly greater proportion of patients receiving luspatercept vs placebo achieved ≥75% reduction in RBC transfusion burden over ≥24 weeks, both overall (34.6% vs 6.6%; P < .001) and among patients with HTB (18.2% vs 3.0%) and LTB (47.1% vs 9.3%) (Figure 2A). The clinical benefit of luspatercept to HTB patients is further supported by these data, indicating that focusing exclusively on TI as an outcome can prevent recognition of significant benefits of many therapies, including luspatercept.
Overall, more patients receiving luspatercept reported a serious adverse event of any grade during weeks 1 to 48 vs placebo (46.4% vs 32.0%, respectively), which was higher than the rates for the luspatercept arm in the primary analysis (31% vs 30% for luspatercept vs placebo).14 Rates were comparable between LTB and HTB patients within the luspatercept arm (47.1% vs 45.5%, respectively). The most common treatment-emergent adverse events (TEAEs) of any grade with luspatercept were fatigue (30.1%) and diarrhea (28.1%) (Figure 2B). Among luspatercept HTB patients, the most common TEAEs were fatigue (25.8%) and peripheral edema (25.8%), whereas among LTB patients, they were diarrhea (37.9%) and fatigue (33.3%) (Figure 2B). Additional efficacy and safety data are presented in the supplemental material.
One limitation, common to long-term follow-up reports of any randomized trial in which clinical outcomes are improved in the intervention arm, is the higher rate of double-blind treatment discontinuation owing to lower efficacy in the control arm. As time progresses, this causes increasing imbalance and may introduce confounders and bias in interpretation of differences in results between the arms. Despite this limitation, more than a quarter of patients randomized to luspatercept remained on treatment for >2 years, supporting its long-term benefit.
In conclusion, luspatercept had a generally acceptable and predictable safety profile while affording sustained periods of TI and effectively reducing transfusion burden among HTB and LTB patients, contributing to maintaining or improving patient quality of life.18 These data further support the sustained clinical benefits of luspatercept in patients with LR-MDS-RS compared with outcomes from the primary analysis.14
Acknowledgments
The authors thank James Matthews of Excerpta Medica, funded by Bristol Myers Squibb, for writing and editorial assistance.
This study was supported by Celgene, a Bristol-Myers Squibb Company, in collaboration with Acceleron Pharma Inc, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA and by Bloodwise UK grants 10024 and 14017 (G.J.M.) and Cancer Research UK grant A22324 (G.J.M.).
Authorship
Contribution: A.M.Z., U.P., G.G.-M., M.A.S., P.F., A.E.D., P.L.G., M.R.S., J.G.J., A.K.V., G.J.M., R.B., V.S., and R.S.K. enrolled patients to the study, and collected and interpreted data; J.Z., G.Z., and X.H. analyzed and interpreted data; J.K.S., R.I., and J.T.B. interpreted data; and all authors contributed to drafting the manuscript and provided final approval to submit the manuscript.
Conflict-of-interest disclosure: A.M.Z. has received grant support, consulting fees, honoraria, and fees for serving on a clinical trial committee from AbbVie, Bristol Myers Squibb, and Novartis; consulting fees and honoraria from Acceleron Pharma, Agios, Astellas, BeyondSpring, Cardinal Health, Daiichi Sankyo, Janssen, Jazz Pharmaceuticals, Seagen, Syndax, Taiho, and TYME; grant support from ADC Therapeutics, Astex Pharmaceuticals, and MedImmune/AstraZeneca; grant support, consulting fees, and honoraria from Amgen, Aprea, Boehringer Ingelheim, Cardiff Oncology, Incyte, Otsuka, Pfizer, Takeda, and Trovagene; consulting fees, honoraria, and fees for serving on a clinical trial committee from Gilead and Kura; and fees for serving on a clinical trial committee from Geron. U.P. has received grant support, consulting fees, and honoraria from Bristol Myers Squibb and Celgene, a Bristol-Myers Squibb Company; grant support and honoraria from Janssen and Novartis; grant support from Amgen and Merck; and honoraria from Abbvie, Geron, and Takeda. G.G-M. has received grant support and consulting fees from Astex Pharmaceuticals, Bristol Myers Squibb, Genentech, and Helsinn Healthcare; and grant support from AbbVie, Amphivena Therapeutics, Aprea, Bristol Myers Squibb, Curis, Forty Seven, H3 Biomedicine, Janssen, Merck, Novartis, and Onconova Therapeutics. M.A.S. has served on advisory boards for Bristol Myers Squibb, Gilead Sciences, Novartis, and Pfizer. P.F. has received consulting fees from Celgene, a Bristol-Myers Squibb Company. A.E.D. has received honoraria from Bristol Myers Squibb and Novartis; and consulting fees from Taiho and Takeda. P.L.G. has received grant support (paid to Stanford University) from Bristol Myers Squibb. M.R.S. has received fees for serving on a steering committee and fees for serving on a data and safety monitoring board from Bristol Myers Squibb, Geron, Ryvu, Sierra Oncology; consultancy and advisory fees for AbbVie, CTI BioPharma, Karyopharm, Novartis, Ryvu, Taiho, Takeda, and TG Therapeutics; grant funding from ALX Oncology, Astex, Incyte, Takeda, and TG Therapeutics; and holds equity in Karyopharm and Ryvu. J.G.J. has received grant support (paid to Columbia University), advisory board fees, and travel support from AbbVie; grant support (paid to Columbia University) from Arog Pharmaceuticals, Astellas, Forma Therapeutics, Genentech, Gilead Sciences, PTC Therapeutics, and Syros Pharmaceuticals; advisory board fees from AstraZeneca; grant support (paid to Columbia University), advisory board fees, and travel support from Bristol Myers Squibb; grant support (paid to Columbia University) and consulting fees from Daiichi Sankyo; and fees for serving on an endpoint committee from Novartis. A.K.V. has provided consultancy for and received research funding from Bristol Myers Squibb; provided consultancy for and received honoraria from Acceleron Pharma; research funding from Janssen and MedPacto; and currently holds equity in Stelexis (a private company). G.J.M. has received support from Bloodwise UK grants 10024 and 14017, Cancer Research UK grant A22324, Celgene, a Bristol-Myers Squibb Company, and Novartis. R.B. has received honoraria, consulting fees, and research funding from Bristol Myers Squibb; honoraria, consulting fees, and research funding TAIHO; and research funding from Takeda. V.S. has received advisory board fees and lecture fees from Celgene, a Bristol Myers Squibb Company; travel support from Janssen Biotech; advisory board fees from Geron, Gilead, Menarini, Novartis, and Takeda Oncology; and grant support, paid to the University of Florence, from Celgene. J.K.S. reports current employment at and currently holding equity in Bristol Myers Squibb (publicly traded company). R.I. reports former employment at and currently holding equity in Bristol Myers Squibb (publicly traded company); current employment at and currently holding equity in Eli Lilly and Company (publicly traded company). J.Z., G.Z., and X.H. report current employment at Bristol Myers Squibb. J.T.B. reports currently holding equity in Bristol Myers Squibb (publicly traded company); and current employment at and currently holding equity in Acceleron Pharma (publicly traded company). R.S.K. has received advisory board fees from AbbVie, Acceleron Pharma, Bristol Myers Squibb, Geron, Jazz Pharmaceuticals, and Novartis; and fees for serving on a speakers’ bureau from Bristol Myers Squibb and Jazz Pharmaceuticals.
Correspondence: Amer M. Zeidan, Hematology, 333 Cedar St, PO Box 208028, New Haven, CT 06520-8028; e-mail: amer.zeidan@yale.edu.
References
Author notes
∗A.M.Z. and U.P. contributed equally to this study.
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independentresearch/data-sharing-request-process.html.
The online version of this article contains a data supplement.