Abstract
Introduction: While CD19 chimeric antigen receptor T-cell therapy (CD19.CART) has altered the treatment landscape for multiple relapsed/refractory (r/r) B-cell malignancies, over 50% of patients ultimately relapse. To elucidate relapse mechanisms, previous studies have predominantly focused on disease- and treatment-related risk factors, while few studies have looked at systemic, host-intrinsic features. In this context, we recently demonstrated that an increase of metabolically high-risk adipose tissue sites, particularly visceral fat, impacts cytokine release syndrome onset and severity via IL-6 (Dos Santos & Rejeski et al, Haematologica 2022). We therefore hypothesized that body composition and nutritional status may also affect post-CAR-T survival outcomes.
Methods: In this single-center, retrospective observational analysis, we studied patients receiving CD19.CAR-T for r/r B-cell malignancies between 01/2019 and 03/2022 in a real-world setting. We comprehensively analyzed the impact of immunometabolic host features including body composition (BC) measurements on clinical outcomes. We extracted psoas and skeletal muscle indices (PMI/SMI) as well as subcutaneous, visceral and epicardial adipose tissue (SAT/VAT/EAT) distributions from pre-lymphodepletion CT images. We further assessed the laboratory-based immuno-nutritional scores Glasgow Prognostic Score (GPS), CRP/Albumin Ratio and the Prognostic Nutritional Index (PNI). Efficacy outcomes were assessed according to Lugano criteria for B-NHL (LBCL, MCL) and according to MRD status for BCP-ALL. Durable responders were defined as reaching at least a partial remission (PR) or better by day 90, while non-responders had stable disease (SD), progressive disease (PD), or died due to treatment-related causes.
Results: 66 consecutively treated patients were included in this analysis (53 LBCL, 9 MCL, 4 BCP-ALL). The following CD19.CART products were applied: axi-cel in 22 patients, tisa-cel in 25 patients and brexu-cel in 9 patients. Patients received a median of four prior treatment lines, which included a stem cell transplantation in 24 patients. The best 90-day overall response rate was 51.5%, with a complete remission rate of 40.9%. At a median follow-up time of 19 months, median PFS was 3.2 months and median OS 14 months.
Based on the BC analysis, we found that CAR-responders displayed increased visceral adipose tissue deposits compared to CAR-non-responders (VAT: 120 vs. 67 cm2, p=0.01). Furthermore, immuno-nutritional scores (GPS, CRP/Albumin-Ratio, PNI) were significantly altered in non-responding patients. Next, we defined cut-off values for further analyses based on receiver operating characteristics for adipose tissue parameters and immunonutritional scores, and on optimal log-rank test separation for sarcopenia parameters.
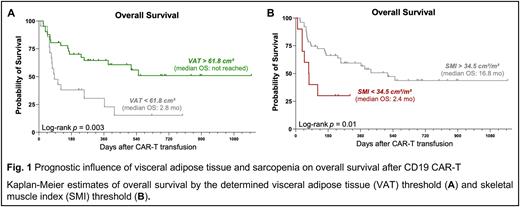
On univariate cox regression analysis, patients with VAT > 61.8 cm² exhibited a significantly lower risk of poor OS (Hazard Ratio [HR] = 0.35, 95% CI 0.18-0.70). Concomitantly, median PFS (5.1 vs. 1.9 months, p=0.04) and median OS (not-reached vs. 2.9 months, Fig. 1A) were significantly extended in patients with excess visceral adipose tissue. On the other hand, a SMI < 34.5 cm²/m², an indicator for sarcopenia, was associated with inferior OS (median 2.4 vs. 16.8 months, Fig. 1B).
In a multivariate analysis adjusting for baseline CRP, LDH and ECOG performance status, increased VAT > 61.8 cm² was associated with improved clinical outcomes (high VAT: adjusted HROS: 0.28, 95% CI 0.13-0.59, p=<0.001). Conversely, sarcopenia significantly increased the risk of poor survival (low SMI: aHROS: 3.63, 95% CI 1.47-8.99, p=0.005). Moreover, malnutrition, defined by a PNI < 39.7, was associated with worse PFS and OS (low PNI: aHROS: 3.27, 95% CI 1.38-7.77, p=0.007).
Conclusions: These findings highlight that body composition and nutritional status play a role as host-intrinsic prognostic factors in the context of CD19.CART. The link between visceral adipose tissue and improved survival suggests that the obesity paradox may extend to patients receiving T-cell based immunotherapies. If validated in future prospective studies, data on body composition and nutritional status may complement existing risk-stratification tools for patients receiving CD19-CART, and may lead to exercise- and diet-based interventional strategies.
Disclosures
Rejeski:Kite/Gilead: Other: Travel Support, Research Funding; Novartis: Honoraria. Buecklein:Miltenyi Biotech: Research Funding; Novartis: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria; Gilead: Honoraria; Pfizer: Research Funding, Speakers Bureau. Blumenberg:Gilead: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Research Funding; Celgene: Research Funding. Schmidt:Bayer: Research Funding; Kite/Gilead: Consultancy, Honoraria, Other: Travel support, Research Funding; BMS: Consultancy, Honoraria, Other: Travel support; Janssen: Honoraria, Other: Travel support; Novartis: Consultancy, Honoraria, Other: Travel support; Takeda: Consultancy. Theurich:Janssen: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; GSK: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Subklewe:Takeda: Other: Travel support; Novartis: Consultancy, Speakers Bureau; Seattle Genetics: Research Funding; Pfizer: Consultancy; Seagen: Research Funding; Morphosys: Research Funding; Miltenyi Biotech: Research Funding; Roche: Consultancy, Research Funding; Bristol-Myers Squibb: Research Funding; Janssen: Consultancy, Speakers Bureau; Gilead: Consultancy, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Speakers Bureau; Amgen: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.