Abstract
Introduction: Lymphodepleting chemotherapy (LDC) is essential for optimal efficacy of chimeric antigen receptor (CAR) T-cell therapy and functions through multiple mechanisms, including elimination of sinks for homeostatic cytokines, such as interleukin [IL]-7 and IL-15. In clinical trials, LDC is typically given on the fifth through third day before cell infusion. However, in real-world practice, cell infusion may be delayed for multiple reasons, including clinical and logistical complications. It remains unclear whether delaying cell infusion impacts clinical outcomes and whether LDC should be repeated if cell infusion is delayed.
Methods: We performed a retrospective cohort analysis of all patients with relapsed or refractory large B-cell lymphoma who received LDC with fludarabine and cyclophosphamide followed by standard of care axicabtagene ciloleucel (axi-cel) at the University of Texas MD Anderson Cancer Center between 1/2018 and 12/2021. For multivariate analyses, only factors significantly associated in univariate analysis were included.
Results: Of 240 patients treated with axi-cel, 40 (16.7%) received delayed cell infusion with a median delay of 2 days (range 1-14 days). Reasons for delay included infections in 34 (85%) patients, disease-related procedures in 3 (7.5%) patients and logistical complications in 3 (7.5%) patients. On univariate analysis, baseline characteristics associated with infusion delay were extra-nodal sites > 1 (p = 0.034), high number of prior therapies (p = 0.015), low absolute monocyte count (p = 0.003), low hemoglobin concentration (p < 0.001), low platelet count (p = 0.009), high C-reactive protein (CRP; p < 0.001), high ferritin (p = 0.01) and high lactate dehydrogenase (LDH; p = 0.016) levels at the time of LDC initiation. On multivariate analysis, the association was maintained only for elevated CRP (odds ratio 1.009; 95% confidence interval [CI] 1.002-1.017; p = 0.009).
When compared to patients who received cell infusion on-time, patients with delayed infusion had similar rates of cytokine release syndrome (CRS) of any grade (90% vs. 93.5%; p = 0.496), grade 3-4 CRS (12.5% vs. 8.0%; p = 0.542), immune effector cell-associated neurotoxicity syndrome (ICANS) of any grade (65% vs. 64.5%; p = 0.952) and grade 3-4 ICANS (42.5% vs. 39.5%; p = 0.860), but exhibited a trend towards increased rate of grade 3-4 cytopenias at day 30 (74.3% vs. 58.0%; p = 0.09). Patients with delayed infusion had a significantly lower day 30 overall response rate (59.0% vs. 79.4%; p = 0.008) but similar complete response rate (43.6% vs. 54.3%; p = 0.293) to those with on-time infusion.
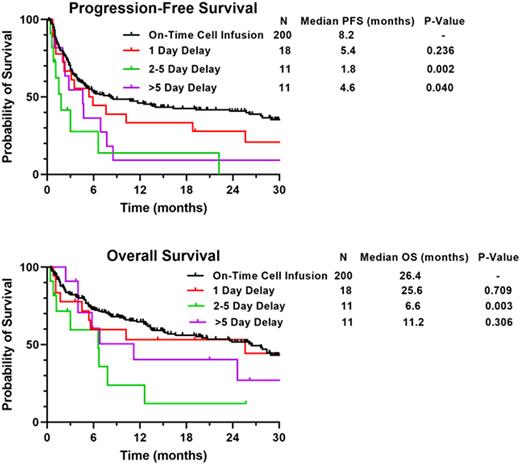
After a median follow-up of 25.7 months (95% CI 22.6-28.8 months), patients with delayed infusion had significantly shorter median progression-free survival (PFS; 3.5 vs. 8.2 months; p = 0.002) and overall survival (OS; 7.8 vs. 26.4 months; p = 0.046) compared to those with on-time infusion. An association between extent of delay and survival was observed, with significantly shorter median PFS in patients who had delay of 2-5 days (1.8 vs. 8.2 months; p = 0.002) and >5 days (4.6 vs. 8.2 months; p = 0.040) but no significant difference in median PFS for patients with a delay of 1 day (5.4 vs. 8.2 months; p = 0.240) compared to those with on-time infusion (Figure).
The association between delayed infusion and shorter PFS was maintained on multivariate analysis including age, International Prognostic Index score, LDH and CRP (hazard ratio 1.567; 95% CI 1.045-2.351; p = 0.03).
When compared to patients with on-time infusion, patients with delayed infusion had lower serum IL-15 concentration (17.0 vs. 26.3 pg/mL) at time of cell infusion but comparable IL-7 concentration (16.4 vs 15.7 pg/mL).
Conclusions: In real-world practice, cell infusion is delayed in more than 15% of patients, mostly due to infectious complications. In this single-center retrospective analysis, cell infusion delay was associated with higher rates of prolonged severe cytopenia and worse clinical outcomes, particularly if delay was longer than 1 day. Larger cohorts are needed to further characterize the impact of delayed cell infusion on treatment efficacy and determine whether biologically rational treatments directed at improving CAR T-cell function in these patients would be beneficial.
Disclosures
Steiner:BMS: Research Funding; Seagen: Research Funding; GSK: Research Funding; Rafael Pharmaceuticals: Research Funding. Nastoupil:ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; Genentech/Roche, MEI, Takeda: Other: DSMC; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding. Hawkins:Bristol Myers Squibb Cell Therapy: Membership on an entity's Board of Directors or advisory committees. Nair:Incyte Corporation: Honoraria. Westin:Kite, a Gilead Company: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Merck: Consultancy; Iksuda: Consultancy; Calithera: Consultancy, Research Funding; MonteRosa: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; MorphoSys/Incyte Corporation: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Abbvie/GenMab: Consultancy; SeaGen: Consultancy. Chihara:Eisai: Honoraria; AstraZeneca: Honoraria. Iyer:Salarius Pharmaceuticals, Inc.: Consultancy. Ahmed:Chimagen: Consultancy, Research Funding; Xencor: Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Tessa Therapeutics: Consultancy, Research Funding; Seagen: Research Funding; Myeloid Therapeutics: Consultancy. Shpall:Navan: Consultancy; NY blood center: Consultancy; Fibroblasts and FibroBiologics: Consultancy; axio: Consultancy; adaptimmune: Consultancy; Bayer: Honoraria; Takeda: Patents & Royalties; Affimed: Other: License agreement. Kebriaei:Ziopharm: Research Funding; Amgen: Research Funding; Pfizer: Consultancy; Kite: Consultancy; Jazz: Consultancy. Neelapu:Bluebird Bio: Consultancy, Honoraria; Unum Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Calibr: Consultancy, Honoraria, Other: Personal fees; Adicet Bio: Consultancy, Honoraria, Other: Personal fees, Research Funding; Legend Biotech: Consultancy, Honoraria, Other: Personal fees; Precision Biosciences: Consultancy, Honoraria, Other: Personal fees, Research Funding; Incyte: Consultancy, Honoraria, Other: Personal fees; Cell Medica/Kuur: Consultancy, Honoraria, Other: Personal fees; Allogene Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Pfizer: Consultancy, Honoraria, Other: Personal fees; Celgene: Consultancy, Honoraria, Other: Personal fees, Research Funding; Novartis: Consultancy, Honoraria, Other: Personal fees; Bristol Myers Squibb: Consultancy, Honoraria, Other: Personal fees, Research Funding; Merck: Consultancy, Honoraria, Other: Personal fees, Research Funding; Kite: Consultancy, Honoraria, Other: Personal fees, Research Funding; Medscape: Consultancy, Honoraria; Aptitude Health: Consultancy, Research Funding; Bio Ascend: Consultancy, Honoraria; Poseida: Research Funding; Cellectis: Research Funding; Karus Therapeutics: Research Funding; Acerta: Research Funding; Takeda Pharmaceuticals: Patents & Royalties: related to cell therapy.. Strati:ADC Therapeutics: Consultancy, Research Funding; TG Therapeutics: Consultancy; Kite Gilead: Consultancy; Astrazeneca Acerta: Research Funding; ALX Oncology: Research Funding; Sobi: Research Funding; Hutchinson MediPharma: Consultancy; Roche Genentech: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.