Abstract
Introduction: The National Heart, Lung, and Blood Institute (NHLBI) recommends hydroxyurea (HU) be offered to all children with sickle cell anemia (SCA) age >9 months via shared decision-making (SDM). However, HU remains underutilized, largely due to parental concerns about safety and potential long-term effects. SDM was recommended because it can decrease parental uncertainty as families clarify their values/preferences and understand scientific evidence about HU. Funded by the Patient-Centered Outcomes Research Institute (PCORI), the Engage HU study (NCT03442114) compared two methods for optimizing SDM for HU in young children with SCA: the American Society of Hematology's (ASH) HU clinician pocket guide (usual care condition) and a HU SDM toolkit (toolkit condition) created by the research team. This study aimed to compare the impact of these on caregiver decisional uncertainty and caregiver perception of clinician use of SDM.
Methods: Engage HU was a prospective, multi-site intervention trial with a unidirectional crossover design in which each clinic began enrolling in the usual care condition and then one-by-one crossed over to the toolkit condition. Inclusion criteria included children ages 0-5 years followed at 12 SCA centers in the US who were candidates for HU and whose caregivers had not decided about HU therapy within 3 months prior to enrollment. After receiving a flyer/letter, eligible caregivers were approached in clinic or by phone. Caregivers completed an e-consent form. Study visits were conducted in-person or via telehealth. Primary endpoints were caregiver decisional uncertainty (Decisional Conflict Scale-DCS) and caregiver perception of clinician use of SDM (Dyadic OPTION scale-OPTION). Lower scores on the DCS indicate less decisional uncertainty and higher scores on the OPTION indicate that the clinician demonstrated more SDM behaviors (e.g., asked about caregiver preferences/values). All survey data were collected using paper surveys or electronically via REDCap™. Data were analyzed using linear regression models in Stata version 17 for each main outcome of interest (DCS and OPTION Total Scores). Our primary predictor was group (toolkit versus usual care). The model was estimated using full information maximum likelihood estimation on the full analysis sample (N=132) to account for missing data. We also accounted for site-level variation, when the sites crossed from usual care to toolkit (i.e., the cluster), when data were collected (0=pre-COVID, 1=during COVID), and the impact of COVID-19 on caregivers (assessed using the COVID-19 Exposure and Family Impact Survey [CEFIS]).
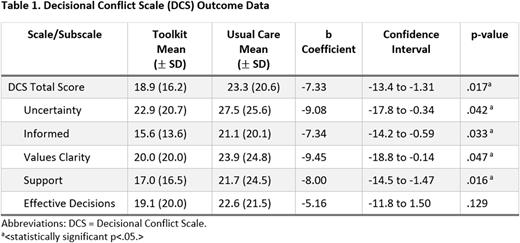
Results: A total of 176 caregivers of eligible children consented. Twelve SCA clinical sites participated; however, four sites were terminated early due to staff turnover or low accrual. Mean caregiver age was 30.19 (SD=6.97); most identified as female (93.1%), Black/African American (89.3%), or Mixed race (6.1%), and mothers (90.8%). Most children had HbSS (94.7%). Preliminary analyses were conducted on 132 caregivers with complete data (n=85 Usual Care; n=47 Toolkit). Regression analyses showed that participants in the toolkit condition had significantly lower DCS Total scores (i.e., lower decisional conflict) than those in usual care condition (b=-7.33, 95% CI=-13.3 to 1.31, p=.017). In addition, significant differences were found on the DCS subscales (see Table 1). Similarly, participants in the toolkit condition had significantly higher OPTION Total scores (i.e., parents reported that the clinician exhibited more SDM behaviors) than those in usual care (b=2.38, 95% CI=0.44 to 4.34, p=.016).
Conclusions: Use of the HU toolkit compared to the usual care approach significantly improved SDM between providers and caregivers of children with SCA; however, given site team turnover, lower than expected accrual, and study interruption due to the COVID-19 pandemic, additional research is needed to confirm these preliminary findings. Future studies should also seek to identify context-specific factors (e.g., clinic processes) that are key to successful implementation of SDM with both younger and older children with SCA.
Disclosures
King:Global Blood Therapeutics: Consultancy, Research Funding. Quinn:Aruvant: Research Funding; Dispersol Technologies: Honoraria; Emmaus Medical: Research Funding; FORMA Therapeutics: Honoraria; Novo Nordisk: Honoraria. Brinkman:Pfizer: Other: Stock in publicly traded company; Merck: Other: Stock in publicly traded company; Abbott Laboratories: Other: Stock in Publicly traded company; Viatris: Other: Stock in Publicly traded company; Johnson & Johnson: Other: Stock in Publicly traded company. Thompson:Agios: Consultancy; Beam: Consultancy; bluebird bio, Inc.: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; CRISPR/Vertex: Consultancy, Research Funding; Baxalta: Research Funding; Biomarin: Research Funding; Editas: Research Funding; Global Blood Therapeutic: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding. Smith-Whitley:Global Blood Therapeutics: Current Employment, Current equity holder in private company. Badawy:Global Blood Therapeutics: Consultancy; Forma Therapeutics: Consultancy; CHIESI Farmaceutici S.p.A: Consultancy; Bristol-Myers Squibb (BMS): Consultancy; Vertex Pharmaceuticals Inc: Consultancy; Sanofi: Consultancy; Bluebird Bio Inc: Consultancy; Pfizer Inc: Research Funding. Treadwell:Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees. Crosby:Global Blood Therapeutics: Honoraria; Forma Therapeutics: Consultancy, Honoraria, Other: Grant reviews.
OffLabel Disclosure:
Hydroxyurea is FDA approved for children with sickle cell disease age 2 and older, but NHLBI guidelines recommend offering it to children as early as 9 months of age.
Author notes
Asterisk with author names denotes non-ASH members.