Abstract
Background Complement-mediated (CM) thrombotic microangiopathy (TMA), also known as atypical hemolytic uremic syndrome, is a serious and potentially life-threatening disorder. Anti-C5 monoclonal antibodies (aC5-mab) reduce the morbidity and mortality. Experience outside clinical trials has been limited due to the rarity of the condition.
Aims To examine the outcomes of patients diagnosed with CM-TMA treated outside clinical trials.
Methods Retrospective, single-institution study. The inclusion criteria required a diagnosis of CM-TMA (laboratory values consistent with TMA syndrome or TMA on a biopsy, with normal ADAMTS13 and absent shiga toxin) and available 6 month follow up data. Day 1 refers to the first day of therapy with aC5-mab.
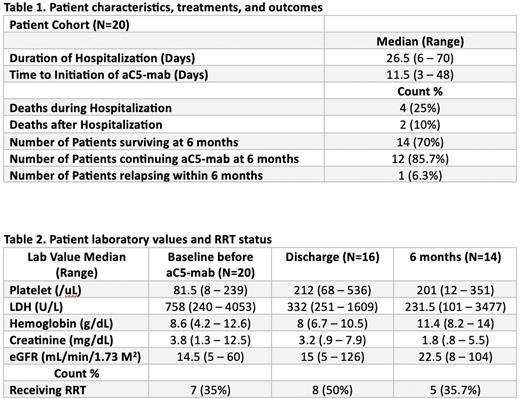
Results Twenty subjects met our inclusion criteria for this report. All were hospitalized during their initial diagnosis. Four (20%) passed away during the hospitalization, while another 2 (10%) died after hospitalization but before 6 months. Fourteen individuals made it to the 6 month follow up. Initiation of aC5-mab occurred at a median of 11 (3 to 48) days after the initial TMA Event. All patients received aC5-mab while hospitalized.
Median laboratory values prior to aC5-mab demonstrated: platelets 81.5/uL, LDH 758 U/L, Cr 3.8 g/dL, and hemoglobin 8.6 g/dL. Median values for those who died during hospitalization and before 6 months were: platelets 79/uL, LDH 1044 U/L, Cr 2.1 g/dL, and hemoglobin 9.1 g/dL. Median values at discharge for surviving patients during hospitalization were: platelets 212/uL, LDH 332 U/L, Cr 3.2 g/dL, and hemoglobin 8.0 g/dL. Median values at 6 months were: platelets 201/uL, LDH 231.5 U/L, Cr 1.8 g/dL, and hemoglobin 11.4 g/dL.
Renal replacement therapy (RRT) was administered to 17 subjects while receiving aC5-mab, with 7 patients receiving RRT prior to admission. At hospital discharge, 8 patients were on RRT, and at 6 months, 5 individuals remained on RRT. Five (25%) patients had kidney transplants prior to the diagnosis of CM-TMA.
Six subjects died, 4 during the hospitalization that led to the diagnosis of CM-TMA and 2 after discharge. The causes of death during hospitalization were severe pneumonia (Day 4), multi-organ failure from lupus flare (Day 6), acute hypoxic respiratory failure from pulmonary hypertension (Day 24) and aspiration pneumonia (Day 47). After hospital discharge, 1 individual who had completed 4 weekly doses of aC5-mab passed away due to CMV pneumonia and sepsis 1 month later. Another patient expired 5 weeks after discharge due to sepsis from an intra-abdominal infection four weeks after the last dose of aC5-mab.
Fourteen subjects remained alive at 6 months; 12 continued aC5-mab and 2 had stopped it after 4 and 5 months of therapy with no complications at 6 months.
Conclusion This is a real-world study of patients with newly diagnosed CM-TMA treated with aC5-mab that provides 6-months follow-up data. Our findings confirm the efficacy of aC5-mab at treating CM-TMA. One of the most important findings from this study includes the description of 6 patients that died after initiation of therapy with aC5-mab. This might be due to our aggressive use of aC5-mab in critically ill patients (selection bias). In these patients the laboratory criteria recommended to diagnose CM-TMA can overlap with other conditions leading to significant variability in the management. Limitations to this study include the retrospective nature and sample size. Further studies to assess the use of aC5-mab in critically ill patients and criteria to define the subjects unlikely to benefit from aC5-mab are urgently needed.
Disclosures
Laber:Alexion Pharmaceuticals: Honoraria, Speakers Bureau.
Author notes
*Asterisk with author names denotes non-ASH members.