Abstract
Introduction:
The current standard-of-care (SOC) cytogenetic techniques for the analysis of Chronic lymphocytic leukemia (CLL) include karyotyping, FISH, and Chromosomal Microarray (CMA), which are labor-intensive, time and cost-prohibitive, and often do not reveal the genetic complexity of the tumor, demonstrating the need for alternative technology for better characterization of these tumors.
Methods:
In this retrospective study, we evaluated 70 CLL samples that were received in our laboratory for clinical testing with either Karyotyping, FISH, and/or CMA. The structural variations (SVs) were filtered against >300 healthy controls (assessed with OGM) to enrich for rare variants, and against genes/loci included in the NCCN and NHS guidelines for hematological malignancies. After concordance assessment, additional clinically relevant information was evaluated on SVs included in NCCN/NHS guidelines or where OGM changed the interpretation of an event that was previously impacting a gene/loci in NCCN guidelines.
Results:
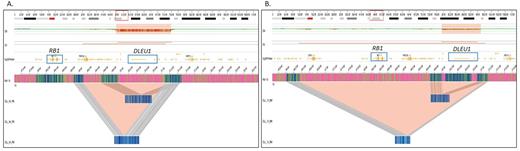
In addition to achieving a high concordance with SOC methods, OGM detected several additional SVs including translocations and copy number variations (CNVs). Importantly, OGM detected several additional clinically relevant SVs listed in NCCN/NHS guidelines. Notably, OGM was able to delineate the 13q14 deletions that harbored only miR15a/16-1 from those that encompassed both miR15a/16-1 and the RB1 genes. The deletion of the RB1 gene along with miR15a/16-1 has been associated with poor prognosis, while the deletion of miR15a/16-1 deletion alone reflects a good prognosis in CLL cases.
Conclusion:
OGM provides a genome-wide analysis that reveals a simple or complex profile along with 13q14 deletion, which is of high significance, as a complex profile is associated with poor prognosis in CLL, and required additional methods such as karyotyping and CMA. OGM alleviates the need for multiple technologies, and can better assist the physician in correlating the genetic profile with disease progression/response to therapy compared to a targeted approach, and exemplifies its utility compared to SOC methods.
Disclosures
Sahajpal:Bioanano INc: Current equity holder in publicly-traded company. Kota:Ariad: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Xcenda: Honoraria; Pfizer Inc: Honoraria, Research Funding. Kolhe:Illumina: Research Funding; Bioanano INc: Honoraria, Research Funding, Speakers Bureau; PGDx: Honoraria, Research Funding; Qiagen: Honoraria, Research Funding; Cepheid: Honoraria; Agena: Honoraria, Research Funding; Perkin Elmer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.