Abstract
Donor-derived leukaemia is a rare outcome in patients following allogeneic hematopoietic cell transplant (HCT) accounting for ~ 0.1-5% of relapsed cases although the true incidence is likely underestimated.
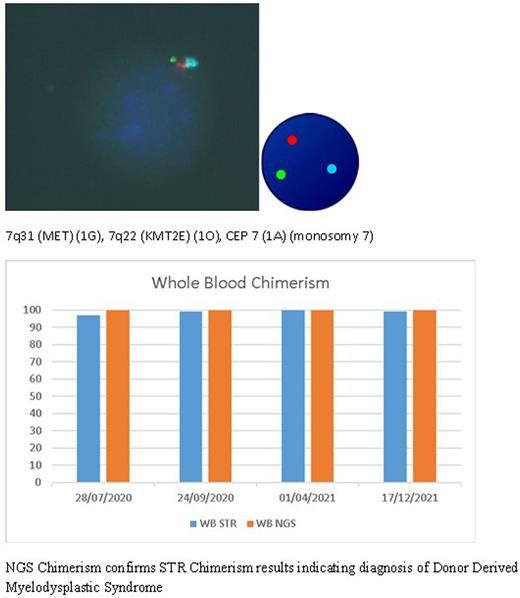
A 39-year-old male patient with a history of ulcerative colitis treated with mercaptopurine was subsequently diagnosed with therapy related acute myeloid leukaemia (AML) and karyotype showed 45,XY,-7. The patient achieved remission after two cycles of treatment and proceeded to a paternal haploidentical HCT. Complete remission with no detectable disease by flow cytometry and fluorescent in-situ hybridisation (FISH) was achieved by day 40 of transplantation with whole blood and T-cell chimerism of 97% as assessed by routine short tandem repeat (STR) chimerism analysis. With withdrawal of immunosuppression this increased to 100% in both fractions. At 11 months post-transplant evidence of low-level cytogenetic relapse emerged with monosomy 7 seen by FISH in 15/200 interphase cells. Chimerism remained stable at 99% with aspirate findings indicating 2-3% blasts and some trilineage dysplasia. Two subsequent marrows showed persistence of monosomy 7 by FISH with full donor STR chimerism.
Although a possibility of donor derived myelodysplastic syndrome (DD-MDS) was considered, due to the monosomy 7, donor lymphocyte infusion (DLI) therapy was administered at 16 and 19 months post-transplant. Monosomy 7 persisted on FISH and standard cytogenetic analysis. To confirm validity of STR chimerism and to distinguish between DD-MDS and relapse of original disease, HLA typing by next generation sequencing (NGS) to exclude HLA loss related relapse and potentially guide rescue decisions was performed alongside NGS chimerism assessment. NGS chimerism utilises genetic markers with population independent discriminative power distributed across 17 chromosomes and further selected to allow sensitive detection combined with accurate and precise quantification of mixed chimerism. NGS chimerism is demonstrated to be sensitive enough to detect a minor DNA contributor comprising down to 0.5% of the sample, detection of low level mixed chimerism and therefore clinical utility in better predicting relapse compared to conventional techniques. Original stored donor cells were tested and showed no evidence of monosomy 7 by FISH.
After confirming DD-MDS a second HCT was considered. Originally unsuccessful unrelated donor search was re-opened and wide family donor search initiated. With azacytidine therapy the patient achieved a short lived cytogenetic remission, but subsequently relapsed and progressed to AML with karyotype of 45,XY,-7,der(16)t(11;16)(q13;p13.1)[9]/46,XY[1] and died 33 months after HCT prior to initiation of a second HCT.
This case was complicated by the presence of monosomy 7 detected by karyotyping in both the initial diagnostic t-AML and in the DD MDS. Monosomy 7 is the most common abnormality found in DD-MDS but a review of the literature available does not seem to indicate any cases where it was also present in the patient's initial diagnostic sample as a separate disease entity. The pathogenesis of DD-MDS remains unclear.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.