Abstract
Background The possibility of treatment discontinuation (TD) in patients with Chronic Myeloid Leukemia (CML) achieving a sustained deep molecular response (DMR) became a reality in clinical practice and is recognized as one goal in the management of the disease. TD is considered successful if at least a major molecular response (MMR or MR3) is maintained. TD is generally considered safe, since MMR is achieved in almost all cases when TKI re-initiation is required.
Progression to accelerated phase/blast crisis (AP/BC) after TD was considered virtually impossible for a long time. However, recent reports documented at least 8 cases of disease progression (DP) after TD, some of which were fatal. These observations raise concerns about the global safety of TD, since this practice can no longer be considered completely immune from the risk of disease progression, which drastically changes the patient situation and perspective. To obtain a numerical assessment of the risk of DP in patients (Pts) with durable DMR, independently of their decision to start TD, the TFR-PRO study was designed.
Study Design and Methods This is a multicentre retro-prospective observational study which monitors CP-CML patients with stable and DMR followed at 20 centres in 5 different countries (Italy, Canada, Germany, Spain, France).
Pts eligibility includes a history of at least 4 years of TKI treatment and at least 18 months of stable DMR, defined as a BCR-ABL1/ABL1 transcript level ratio lower than 1/10,000.
The primary objective is to assess the risk of progression to AP/BC, expressed as time adjusted rates (TAR), and its relation to TD in the population of CML Pts who underwent a first or subsequent TD. Eligible Pts who did not discontinue TKI treatment are considered as a benchmark group. A flexible statistical approach allows to attribute Pts to the two groups according to their decision about TKI discontinuation (TD yes or no).
Secondary endpoints are TAR of molecular relapse (MR, i.e. loss of MMR), Relapse Free Survival (RFS, defined as the time between eligibility or TD and MR), progression free survival and overall survival.
Results The study opened on July 24th, 2020. As of July 24th 2022, 810 Pts were registered, of whom 778 patients are presently eligible, for a total of 3949 person years available for analysis. Median follow up is 6.0 years (range 0.2-15 years), median age at diagnosis was 50 years and 45% Pts are female. Sokal score (available in 406 patients) was low in 47.54%, intermediate in 33.25% and high in 19.21%.
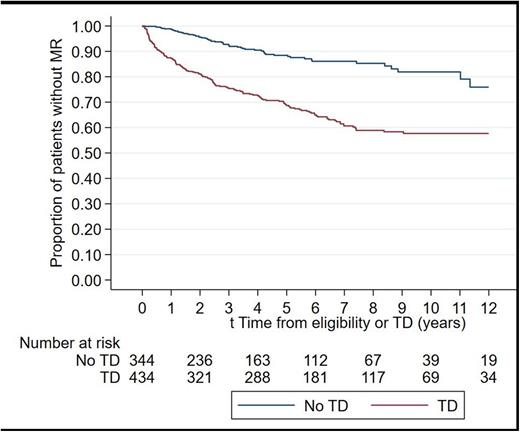
A total of 434 Pts (56%) attempted TD at some point; 35 Pts (4.40%) attempted TD twice. MR occurred in 154/434 (35.5%) Pts after TD for a TAR of 12.9/100 person years, (95% confidence interval (CI) [11-15.1]), but also in Pts who did not attempt TD (61/344, or 17.7%), with a TAR of 2.22/100 person years (95%CI [1.7-2.8], p<0.0001. A graphical representation of the estimated proportion of Pts with MR is provided in figure 1.
One patient progressed to lymphoid BC 18 months from TD; he received allogeneic BMT and is presently in remission. Thus the risk of progression is 1/778 or 0.13% (95% CI [0.003-0.7]) in the entire cohort; this value goes from 1/434 or 0.23%, (95% CI 0.006%-1.3]) for Pts who attempted TD, to 0% (95% CI [0%-0.872%]) for Pts who did not attempt TD. The TAR of progression is 0.026/100 person years (95%CI [0.004-0.184]) in the entire cohort; this value ranges from 0.084/100 person years (95%CI [0.012-0.593]) for Pts who attempted TD to 0/100 person years (95%CI [0-0.11]) for Pts who did not attempt TD.
Conclusions These results obtained in a real world scenario indicate that slightly more than half of 778 eligible Pts attempt TD. Pts who discontinue treatment have a significantly higher risk of losing MR3 than Pts who do not, although MR happen over time also in patients who do not discontinue, and the overall rate of MR after TD is lower than in previous studies. The risk of progression to AP/BC is estimated as 0.13% or 0.026/100 person years; this value is apparently higher for Pts who attempted TD but the 95% CI of the two groups overlap, indicating a lack of significant difference. We conclude that CML progression in Pts eligible for TD is a rare event (approximately 1 in 1000 patients or 0.026% per year in a single patient) and that its relationship with TD is possible but not proven. Additional data including 155 patients already registered but not yet analysed will be presented. Future studies will be needed to confirm our results.
Disclosures
Assouline:Genentech/Roche, Astra Zeneca, Novartis, BMS, Jazz, Gilead, Amgen, Beigene, Abbvie, Paladin: Consultancy, Honoraria; Novartis: Research Funding. Nicolini:Incyte biosciences: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis Services, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; KARTOS: Consultancy; Sun Pharma Ltd: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees. Abruzzese:BMS, Incyte, Novartis, Pfizer: Consultancy. Elena:Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees. Galimberti:Abbvie Incyte, Novartis, Janssen, Astrazeneca, Pfizer: Honoraria. Stagno:Celgene, Incyte, Novartis, Pfizer: Honoraria, Speakers Bureau. Saussele:Incyte: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Roche: Honoraria; Pfizer: Honoraria. le Coutre:Pfizer: Honoraria; Novartis: Honoraria; Incyte: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.