Abstract
Introduction. Protein arginine methyltransferase 1 (PRMT1) transfers a methyl group to the targeted arginine residues on a variety of proteins that regulate cellular function and proliferation. ADAMTS13 protease is significantly modified at post-translational levels that might alter the protein secretion and function. Deficiency of plasma ADAMTS13 activity may result in thromboinflammatory diseases such as thrombotic thrombocytopenic purpura (TTP). However, whether PRMT1-mediated arginine methylation on ADAMTS13 protein affects its secretion and function is unknown.
Methods. The number of arginine on ADAMTS13 predicted to be modified by methylation was assessed with a MeMo web tool. Additionally, arginine methylation was blocked with a type 1 methyltransferase inhibitor (MS023) on recombinant full-length ADAMTS13 and a truncated MDTCS protein expressed in stably transfected HEK293 cells or endogenous full-length ADAMTS13 in human hepatic stellate cells (LX-2). The ADAMTS13 or variant protein and proteolytic activity in cell lysate and conditioned medium were determined by immunoblotting and FRET-vWF73 assay, respectively.
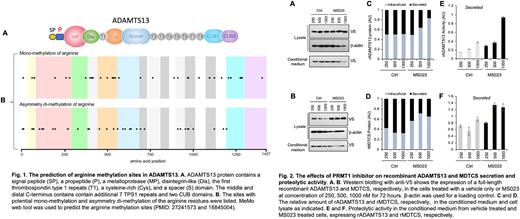
Results. Approximately 35 asymmetry di-methylated arginines and 35 mono-methylated arginines may be present on ADAMTS13 protein (Fig. 1). The arginine sites in the CUB domains and spacer domain are predicted for modification with mono-methylation and asymmetry di-methylation, respectively. Treatment of HEK293 cells expressing recombinant full-length ADAMTS13 (Fig. 2A and 2C) and MDTCS (Fig. 2B and 2D) with the MS023 that blocks type 1 methyltransferase enzymatic activity resulted in dramatically reduced protein secretion. Interestingly, the secreted recombinant full-length rADAMTS13 (Fig. 2E) and MDTCS (Fig. 2F) proteases exhibited an enhanced proteolytic activity towards a FRETS-VWF73 substrate. Additionally, treatment of LX-2 cells expressing native ADAMTS13 with MS023, proteolytic activity of the secreted ADAMTS13 was also increased in a concentration-dependent (not shown).
Conclusions. Our results demonstrate for the first time that arginine methylation may occur abundantly in full-length ADAMTS13 protein and such a modification may affect ADAMTS13 biosynthesis and secretion, but paradoxically enhance the enzymatic activity of secreted ADAMTS13 protease. Our ongoing study is to determine the exact sites of arginine methylation using mass spectrometry and the molecular mechanism underlying how such a post-translational arginine methylation alters ADAMTS13 enzymatic activity. The findings may change how we treat TTP and other thromboinflammatory diseases.
Key words: ADAMTS13, PRMT1, thrombotic thrombocytopenic purpura (TTP), post-translational modification, and protein secretion.
Disclosures
Zheng:Alexion: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.