Abstract
Background BIA-ALCL is a rare form of CD30-positive, ALK-negative ALCL associated with textured breast implants. Patients with stage I BIA-ALCL have disease limited to the capsule which can be completely resected to achieve cure. Advanced stages of disease with capsular invasion, lymph node involvement and/or distant spread require more therapeutic interventions, including radiation (XRT), chemotherapy (CTX), or immunotherapy (ADC). Chemotherapy treatments are similar to those for systemic ALCL and often include anti-CD30 ADC brentuximab vedotin (BV). This study aims to compare outcomes between patients (pts) with advanced BIA-ALCL treated with adjuvant CTX and BV versus those without treatment (Tx).
Methods This study is a single institution, IRB approved retrospective chart review, wherein we examined newly diagnosed pts with BIA-ALCL and review of literature between 2008 and 2022. Variables collected included age, radiologic imaging, stage, surgical procedure, pathology review, and laboratory data. Data on treatment and progression, including response to frontline CTX and BV-based therapy, was also analyzed. Overall survival (OS) was the primary endpoint of the study. R studio version 4.2.1 was used for statistical analysis.
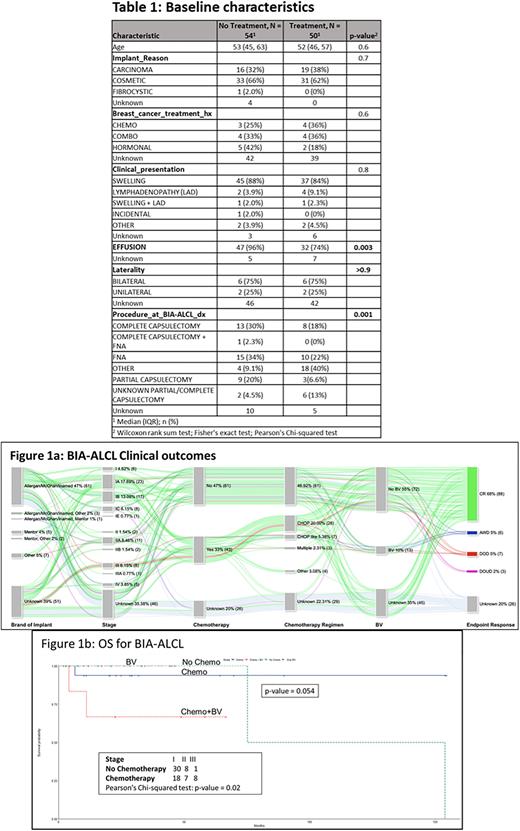
Results In a cohort of 130 pts (71 MDACC pts and 59 literature review pts) with BIA-ALCL, the median age was 53 years (range, 29-77). The indication for implants were as follows: 69 (53%) for cosmetic purpose, 45 (35%) for breast reconstruction following breast cancer, and 1 due to fibrocystic changes. Twenty-five pts with a history of breast cancer had a recorded history of Tx including CTX, radiation, and hormonal therapy. There were 61 pts (47%) with silicone implants, 30 (23%) with saline, and 3 (2%) had both silicone and saline; implant filling was not available for 36 pts (28%). In terms of types of implants: 61 (47%) had Allergan/McGhan/Inamed (A/M/I), 3 (2%) had A/M/I and implants from other companies, 1 had A/M/I and Mentor (1%), 5 (4%) had Mentor, 2 (2%) had Mentor and other companies, and 7 (5%) had implants from other companies; data was not available for 51 (39%) pts. All 82 pts for whom data was available had textured implants. The average time between implant placement and diagnosis was 12.8 years. On initial clinical presentation, 97 (75%, n=130) pts had swelling, 95 (73%, n= 130) had effusions, 22 (17%, n=130) had lymphadenopathy (LAD), and 7 (5%, n=130) had other clinical presentations such as contractures and rash. Stage included: I- 42%, II- 12%, III-7%, and IV-4%. Overall, 54 (52%) pts did not receive Tx (CTX and BV) and 50 (48%) pts did. Among pts that received no treatment (n=54), 96% had effusions and among pts who had Tx (n= 50), 74% had effusions (p=0.003). Fewer pts on the Tx arm underwent capsulectomy versus those who did not receive Tx: 17 (37.6%) vs 25 (56.8%), p= 0.001). For CTX pts 86% (n=43) pts received CHOP or CHOP-like regimens. Seven (14%) received BV only while 6 (12%) had CTX + BV. A median of 5.1 months passed between diagnosis of BIA-ALCL and starting CTX. Eighty-eight (68%) pts achieved complete remission, 6 (5%) pts were alive with disease, 7 (5%) pts died from disease and 3 (2%) pts died from unrelated diseases. Endpoint response was not available for 26 pts. Most pts who did not receive CTX had Stage I and were treated with complete capsulectomy. We compared the overall survival amongst pts with no Tx, CTX, CTX + BV, and BV. The survival probability of pts receiving CTX and CTX + BV was lower (0.9 and 0.63, respectively) as the disease was similar to systemic ALCL. Overall, there was no significant difference in OS rate (p=0.054) among all four groups of pts.
Conclusion Complete surgical excision continues to play a central role in the management of pts with BIA-ALCL. Although most pts with BI-ALCL have excellent outcomes, a subset of pts with advanced stage disease has a more aggressive course and need CTX or BV. Given the smaller numbers of pts with various CTX combinations, prospective studies are required to evaluate the beneficial role of BV in pts with advanced BIA-ALCL.
Disclosures
Nastoupil:BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding; ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; Genentech/Roche, MEI, Takeda: Other: DSMC. Fowler:Celgene: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Roche: Consultancy, Research Funding; BostonGene Corporation: Current Employment, Other: Leadership. Ahmed:Servier: Membership on an entity's Board of Directors or advisory committees; Chimagen: Consultancy, Research Funding; Xencor: Research Funding; Tessa Therapeutics: Consultancy, Research Funding; Seagen: Research Funding; Merck: Research Funding; Myeloid Therapeutics: Consultancy. Nair:Incyte Corporation: Honoraria. Pinnix:Merck Inc: Research Funding. Hunt:Armada Health: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Cairn Surgical: Research Funding; Eli Lilly & Co: Research Funding; Lumicell: Research Funding. Iyer:Salarius Pharmaceuticals, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.