Abstract
BACKGROUND: The advent of several new drugs is spearheading a paradigm shift in the treatment of elderly patients with diffuse-large-B-cell-lymphoma (DLBCL), towards new combinations, devoid of intravenous chemotherapy (i.e. chemo-free). Herein, we report the updated results, of an all-oral metronomic schedule (mCHEMO), used upfront in elderly/Frail DLBCL patients. We also outline the results of an in-vitro study, which assessed the activity of mCHEMO in DLBCL cell lines.
METHODS & PATIENTS: Data were retrospectively collected from four clinical centres. The mCHEMO schedule, termed R-DEVEC, is based on: cyclophosphamide(CTX) 50mg, administered daily for up to 21-days; Vinorelbine(VNR) 30mg administered three days a week; +/- Etoposide(ETO) 50mg, administered daily for up to 14-days; Prednisolone 25mg on alternate days, + iv/sc Rituximab). Patients who: 1) were Very elderly/Frail without a care-giver, 2) had CNS localization; 3) had dysphagia, were not eligible for this treatment. A pharmacological study to assess the in-vitro activity of metronomic doses of ETO, VNR and their combinations, was performed in three different DLBCL cell-lines.
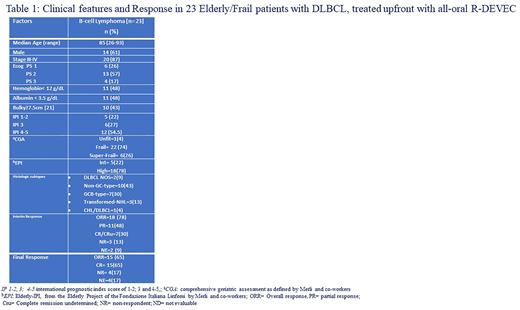
RESULTS: Twenty-three DLBCL, with a median age of 85 years (age-range=26-93 years; 78% ≥80 years), started the R-DEVEC upfront. Based on the comprehensive geriatric assessment (CGA) 15(65%) subjects scored Frail, seven (41%) Super-Frail and one (5%) Unfit, respectively; While the Elderly-IPI-score (EPI) was high in 18 (86%) and intermediate in 5 (14%) patients, respectively. All patients were considered for outcome and toxicities. Fifteen out of 23 (65%) completed six induction cycles. The remaining eight subjects discontinued for: refractory DLBCL in 4/23(17%), treatment-related serious adverse events (TR-SAE) in 3/23(13%) and unrelated SAE in 1/23(4%). Following a median follow-up of 22 months (range=4-80 months), 15 patients died: seven (47%) for causes unrelated to DLBCL or its treatment, six (40%) for progression and two (13%) for multiorgan failure, following treatment stop. At the end of induction 65% achieved complete remission (CR); Overall and progression-free-survival at 24 months were 56% (95% confidence interval [95CI]=48%-64%) and 70% (95CI=65%-86%), respectively. In Univariate analysis, only subjects with 1) bulky disease >7.5cm, 2) high EPI-score and 3) Super-Frail by CGA score, showed a trend for worse survival. Overall, TR-SAE were five (22%, 95CI=14-33): three febrile neutropenia and two pneumonia, respectively. Following the treatment of the first 12 patients, due to the occurrence of TR-SAE and of Grade≥3 neutropenia and anaemia (41% and 25% respectively), Frail patients started treatment with only seven days of ETO , while in super-Frail subjects it was omitted (i.e. R-DEVEC-light). This allowed to minimize toxicity. The pharmacological study showed both ETO and VNR, given at metronomic doses, had marked antiproliferative activity, in three different DLBCL lines. Furthermore, co-administration of ETO and VNR, showed an enhanced activity.
CONCLUSIONS: the remission-rate and the long-term efficacy of upfront R-DEVEC, is remarkable in this subset. This agreed with in-vitro studies, which showed high activity of mCHEMO. R-DEVEC is a promising, chemo-free, and inexpensive schedule for first-line treatment of elderly/frail DLBCL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.