Abstract
Introduction Currently, ruxolitinib, fedratinib and pacritinib are three FDA-approved JAK2 inhibitors used for palliation of splenomegaly and constitutional symptoms in patients with myelofibrosis. However, a durable survival benefit from JAK2 inhibitor therapy remains unclear. In the current study, our primary objective was to retrospectively compare long-term treatment outcomes and identify predictors of response and survival in myelofibrosis patients treated on ruxolitinib, fedratinib, momelotinib or BMS-911543 JAK2 inhibitor clinical trials.
Methods The current study includes patients with primary myelofibrosis (PMF), post-polycythemia vera and post-essential thrombocythemia MF enrolled on ruxolitinib (NCT00509899), fedratinib (NCT00631462, NCT01420770), momelotinib (NCT00935987, NCT01236638), or BMS-911543 (NCT01236352) clinical trials at the Mayo Clinic between October 2007 and July 2013. Study patients were retrospectively recruited after institutional review board approval with follow-up updated in July 2022. Spleen and anemia response were assessed according to the revised IWG-MRT criteria (Blood, 2013).
ResultsPatient characteristics: A total of 183 JAK inhibitor naïve patients with MF (median age 65 years, range 34-89; 58% males, 60% PMF) received ruxolitinib (n=50, 27%), fedratinib (n=23, 13%), momelotinib (n=79, 43%) and BMS JAK2 inhibitor (n=31, 17%) at a median of 27 months following diagnosis. Driver mutation profile included JAK2 in 80%, CALR in 11%, MPL in 5%; other mutations included ASXL1 in 58/124 (47%), SRSF2 in 16/84 (19%) and IDH1/2 in 2/74 (3%) of evaluable patients. Karyotype was abnormal in 97 (53%); among the latter, 53% were unfavorable. DIPSS-plus risk distribution was intermediate-1 (15%), intermediate-2 (46%) and high (39%). 166 (91%), 66 (36%) and 136 (74%) of patients demonstrated palpable splenomegaly, transfusion-dependent anemia, and constitutional symptoms, respectively.
Response: At a median follow up of 3.7 years (0.1-14.4 years), 178 (97%) patients have discontinued therapy. 3 and 5-year discontinuation rates were 77% and 92%, respectively with median treatment duration of 17 months. Spleen response was achieved in 83 of 166 (50%) evaluable patients with median response duration of 22 months (2-132 months) and more likely with fedratinib (83%) vs ruxolitinib (32%), momelotinib (47%) or BMS-911543 JAK2 inhibitor (62%) (p=0.0003) and in the absence of ASXL1 mutations (58% vs 37%, p=0.02). Treatment with fedratinib vs ruxolitinib (p=0.04) and ASXL1 mutations (p=0.01) remained significant on multivariable analysis. Transfusion independence was achieved in 27 of 66 (47%) transfusion-dependent patients for a median of 14 months (6-82 months), and more likely with momelotinib (51%) vs fedratinib (13%), ruxolitinib (30%) or BMS-911543 JAK2 inhibitor (44%) (p=0.15) and in the presence of SRSF2 mutations (75% vs 31%, p=0.02), the latter retained significance on multivariable analysis (p=0.04).
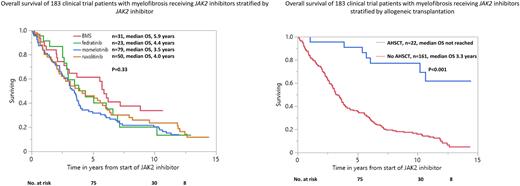
Survival: At last follow-up, 149 (81%) patients have died with median overall survival of 3.7 years (3/5/10-year survival; 60%/41%/16%). Survival was similar with ruxolitinib, fedratinib, momelotinib and BMS-911543 JAK2 inhibitor (4, 4.4, 3.5 and 5.9 years, respectively, p=0.33) but longer in 22 patients that were transplanted vs those not transplanted (not reached vs 3.3 years; p<0.001, 5/10-year survival, 91%45% vs 47%19%) (Figure 1). On univariate analysis limited to non-transplanted patients, survival was superior in DIPSS-plus intermediate-1 risk (7, 3.5, 2.9 years, intermediate-1, 2, high-risk, respectively, p=0.001), with absence of ASXL1 (3.8 vs 2.8 years, p=0.003) and absence of SRSF2 mutations (3.7 vs 3.5 years, p=0.02), and in spleen and anemia responders (5.1 vs 2.3 years, p<0.0001)(3.5 vs 2.1 years, p= 0.003) respectively, the latter three variables remained significant on multivariable analysis.
Conclusions The current study provides long-term outcome data for myelofibrosis patients treated on JAK2 inhibitor clinical trials and underscores the durable survival benefit conferred by allogeneic transplantation. Survival was not influenced by type of JAK2 inhibitor but was superior in spleen and anemia responders and in the absence of SRSF2 mutations. In addition, fedratinib use and absence of ASXL1 mutations predicted spleen response, while SRSF2 mutations correlated with anemia response.
Disclosures
Begna:Novartis: Honoraria; ImmunoGen: Research Funding. Al-Kali:Astex: Other: research support to institution. Litzow:Abbvie: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Syndax: Research Funding; Jazz: Consultancy; Actinium: Research Funding; Pluristem: Research Funding; Biosight: Other: Data Monitoring Board.
Author notes
∗Asterisk with author names denotes non-ASH members.