Abstract
Introduction: Chronic lymphocytic leukemia (CLL) with prolymphocytic progression is a new disease entity defined in the 5th edition World Health Organization (WHO) classification as a CD5+ non-mantle B-cell neoplasm with at least 15% prolymphocytes in the peripheral blood or bone marrow, partially replacing the prior classification of B-cell prolymphocytic leukemia (B-PLL). Due to the rarity of prolymphocytic progression, patient characteristics and disease natural history have not been well-described, particularly in the modern era of novel targeted therapies for CLL. In this nationwide multicenter review, we present an initial retrospective analysis of this newly defined disease classification.
Methods: An electronic query of the Veterans Affairs (VA) Corporate Data Warehouse and retrospective chart review were performed to identify patients who had CD5+ CLL with ≥ 15% prolymphocytes in the blood or bone marrow between the years 2014 (FDA approval of ibrutinib) and 2022. Responses were retrospectively categorized using the International Workshop on CLL (iwCLL) response criteria. Pearson's chi-squared test was utilized for analysis of categorical variables. Survival was measured with the Kaplan-Meier method and analyses were performed using the Wilcoxon test and Cox regression.
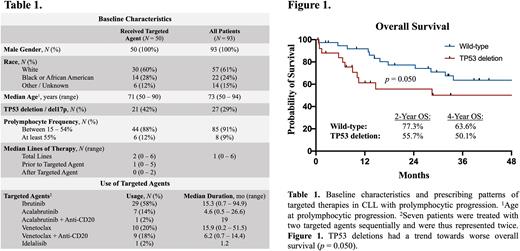
Results: Among 36,973 VA patients with ICD-9/10 codes for CLL between 2014-2022, the electronic query and chart review identified 93 patients (0.3%) from 28 different VA sites who met the 2022 WHO classification for prolymphocytic progression based on morphology (Table 1). The median duration of follow-up from the time of prolymphocytic progression was 28.1 months (range, 0.5 - 121.6). The 2-year and 5-year overall survival (OS) from prolymphocytic progression were 66.2% (95% CI, 55.0 - 75.2) and 35.3% (95% CI, 23.1 - 47.7), respectively. Eight patients (9%) had ≥ 55% prolymphocytes, which had formerly been classified as B-PLL. The median years from diagnosis of CLL to reaching ≥ 15% and ≥ 55% prolymphocytes were 3.7 (range, 0 - 28.4) and 3.2 (range, 0.6 - 10.8), respectively. The % prolymphocytes did not impact OS in a Cox regression model. TP53 deletions were detected in 39% of those with 15 - 54% prolymphocytes compared to 67% in ≥ 55% prolymphocytes (p = 0.22). The detrimental impact of TP53 deletion trended towards significance (2-year OS 55.7% vs. 77.3%, p = 0.050) (Fig 1).
Fifty patients (54%) were treated with oral BTK, BCL-2, or PI3K inhibitors after prolymphocytic progression (Table 1). The most commonly prescribed therapies were ibrutinib (n = 29), venetoclax (n = 19), and acalabrutinib (n = 8). An anti-CD20 monoclonal antibody was combined with venetoclax in 9 patients (18%). The median lines of therapy prior to initiation of a targeted agent was 1 (range, 0 - 5). At the start of therapy, bulky lymphadenopathy, constitutional symptoms, and splenomegaly were reported in 24%, 32%, and 62% of patients, respectively. In addition, 31% had a hemoglobin < 11 g/dL, 40% had a platelet count < 100 x 109/L, and 60% had an elevated LDH. Among evaluable patients, a partial response (PR) by iwCLL criteria was observed in 13 cases (28%), with another 18 (38%) fulfilling the hematologic criteria for PR but lacking follow-up imaging to confirm improvement in splenomegaly or lymphadenopathy. The median progression free survival (PFS) for patients treated with a targeted agent was 31.4 months.
At last follow-up, the median duration of targeted therapy was 13.1 months (range, 0.2 - 94.9), with 18 (36%) remaining on therapy. The most common reasons for discontinuation of therapy were disease progression (n = 14) and drug toxicity (n = 12). After discontinuation of the first targeted agent, 13 patients (26%) received additional treatment, such as another targeted agent (n = 7) or cytotoxic chemotherapy (n = 6). One patient went on to receive an allogeneic stem cell transplantation. There were 22 deaths, with the most common causes being disease progression (n = 11), infection (n = 3), and unknown cause (n = 5).
Conclusion: Prolymphocytic progression of CLL represents a newly defined heterogeneous disease with variable presentations and patient survival. As in CLL, TP53 deletions may carry prognostic value. Though few patients had ≥ 55% prolymphocytes, the percent of prolymphocytes did not appear to influence overall survival in this cohort. Oral targeted agents have efficacy in this population and were used in over half of the patients evaluated.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.