Abstract
Introduction: Myeloma is a plasma cell malignancy in which a high-risk set of patients can be defined by genomic markers including cytogenetic groups such as loss of 1p, gain or amplification (gain/amp) 1q, and TP53 abnormalities. Increasingly, other markers are also being identified that are associated with progression such as PHF19. These markers are highly prevalent at relapse, but can exist in the earlier stages of the disease. Despite these cytogenetic groups being well studied in relation to patient outcomes, there is still not yet a clear association with subclonal structure and subclonal chromatin accessibility.
Methods: Bone marrow aspirates from Smoldering Multiple Myeloma (SMM; n=10), Newly Diagnosed Multiple Myeloma (NDMM; n=22), and Relapsed/Refractory Multiple Myeloma (RRMM; n=17) patients after treatment with Immunomodulatory, proteasome inhibitors, and immunotherapies. All these samples underwent CD138+ sorting and single cell multiomic sequencing (RNA-seq and ATAC-seq; 10X Genomics). The multiomic single cell data were integrated using Seurat via weighted nearest neighbors (WNN). Copy number variants (CNVs) were generated using InferCNV. Cell clusters were evaluated for cytogenetic markers, enrichment in disease progression, gene expression, and other markers of high-risk disease. In total, 335,025 high quality plasma cells were examined across the 49 patient samples.
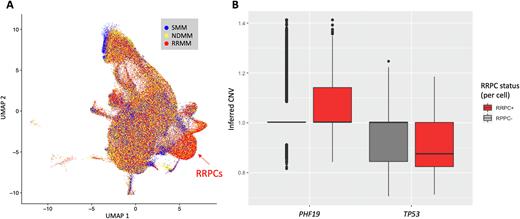
Results: Integration of all 49 patient samples resulted in 25 clusters representing diverse subsets of malignant and normal plasma cells. We identified a subtype of plasma cells that were associated with RRMM disease and were denoted relapse/refractory-associated plasma cells (RRPCs). The relative proportion of this cluster across patients significantly increased from SMM to NDMM (P = 0.025) and from NDMM to RRMM (Fig. 1A, P < 0.001). Patients with gain/amp 1q or TP53 mutations had a greater proportion of RRPCs (P = 0.029 and P = 0.023, respectively). When evaluated further for regulatory mechanisms of cell cycle, the RRPCs had increased expression of PHF19 (Log2FC = 2.79, P < 0.001) and EZH2 (Log2FC = 2.46, P < 0.001), a downstream effector of PHF19, which affects histone methylation and chromatin accessibility.
RNA-seq data generated more distinct clusters per sample compared to ATAC-seq data (3 RNA clusters vs. 2 ATAC clusters, P=0.001). We identified frequent subclonal 1q amplifications and 17p deletions across SMM, NDMM, and RRMM patients. Samples with alterations on 1p, 1q, 8q, 9q, or 11q, had more than one distinct cluster in both the RNA (OR=5.96, P = 0.034) and ATAC data (OR = 12.27, P = 0.001). 1p alterations alone were associated with samples containing more than 2 distinct RNA clusters (OR=5.96, P = 0.034). Furthermore, the number of distinct ATAC clusters correlated with the total number of 1p, 1q, 8q, 9q, and 11q CNV events (PCC=0.35, PCC p-value=0.016). Genomic positions indicated key genes on these chromosomes that correlate with ATAC clustering are TENT5C (1p) and MYC (8q), and PHF19 (9q) as they relate to subclonal chromatin accessibility and disease risk. Notably, PHF19 is located on 9q and had increased copy number for RRPCs compared to other clusters (Fig. 1B, P < 0.001). Likewise, and mirroring the patient level TP53 mutational status, the inferred TP53 CNV status was lower in RRPC cells compared to other clusters (Fig. 1B, P < 0.001). In RRMM patients, inferred CKS1B CNV status was associated with GPRC5D expression (PCC = 0.27, P < 0.001), a potential target for myeloma with gain/amp 1q.
Conclusion: We have generated the largest dataset of CD138+ myeloma cell multiomics to date comprising 335,025 cells across 49 patients at different stages of progression. Based on these data, expression of high-risk markers are largely found in a subset of cells from any given patient and this subset increases during myeloma progression. These cells, denoted RRPCs, have increased expression of PHF19 and EZH2 and are associated with 9q and PHF19 copy number changes which have measurable effects on chromatin accessibility. Furthermore, greater proportions of RRPCs were found in patients with 1q and TP53 alterations and RRPCs tended to have lower inferred TP53 copy number. Using RNA clusters, ATAC clusters, and inferred CNV status, we found that 1p, 1q, 8q, 9q, and 11q alterations accounted for subclonal chromatin accessibility differences and GPRC5D was identified as a potential target for gain/amp 1q clones.
Disclosures
Johnson:Genentech: Research Funding. Chopra:Genentech Inc. (Roche): Current Employment; Roche, BMS, Amgen: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties: Publication number: 20210100805 Publication number: 20200405737 Patent number: 10695352 Patent number: 10653710 Publication number: 20180303840 Publication number: 20180296583. Dos Santos:Genentech: Current Employment, Current equity holder in publicly-traded company. Nixon:Roche / Genentech: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Abonour:Bristol Myers Squibb: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Janssen: Honoraria, Research Funding, Speakers Bureau; Takeda: Honoraria, Research Funding; Amgen: Honoraria; Prothena: Honoraria. Abu Zaid:Pharmacyclics: Research Funding; Janssen: Research Funding; BMS: Research Funding; Genentech: Research Funding; Cormedix: Current equity holder in publicly-traded company; Pieris: Current equity holder in publicly-traded company; Ossium Health: Consultancy. Walker:Genentech: Research Funding; Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.