Abstract
Introduction. URBAN [NCT04034056] is a non-interventional, retrospective/prospective study evaluating effectiveness and safety of obinutuzumab-based treatment in the standard clinical care of patients (pts) with previously untreated advanced Follicular Lymphoma (FL). The study is ongoing at 46 sites in Italy. The objective of this analysis was to evaluate the effects of COVID-19 pandemic in terms of infection rates and severity, outcomes, and effectiveness of vaccination strategies in the study population. The observation period encompasses the obinutuzumab-chemotherapy induction phase, the maintenance phase with bimonthly single-agent obinutuzumab, and 1-year follow-up period afterward.
Methods. Data on COVID-19 vaccination, COVID-19 infections and outcomes (hospitalizations and deaths) were collected up to the cut-off date (CCOD) of 31-Jan-2022 and were analyzed overall, according to vaccination status, COVID-19 pandemic wave, and period. The following periods were considered: 25-Feb-2020 - 01-Mar-2021 (from the outbreak of pandemic up to the start of vaccination campaign for hematological patients), 01-Mar-2021 - 01-Nov-2021 (predominant delta variant of SARS-CoV-2), and 01-Nov-2021 - 31-Jan-2022 (predominant omicron variant). Comparisons of categorical variables were obtained by Chi-square or Fisher exact test.
Results. Information on vaccination status was available for 270 of the 283 enrolled pts who had access to COVID-19 vaccines (BNT162b2, mRNA-1273 or ChAdOx1-S) during the study; 245 (90.7%) of them had received at least one dose of vaccine at CCOD (13.5%, 1 dose; 31.4%, 2 doses; 55.1%, 3 doses). Most of the doses (503/568, 88.6%) were given during obinutuzumab maintenance, since 207 pts had already initiated this phase in March 2021. Fifty-three pts (21.6% of those vaccinated) had at least one cycle delay due to vaccination and all cycle delays occurred during maintenance; no treatment delays were reported during induction.
Median observation time for pts in the "unvaccinated" status (i.e. before vaccination or not vaccinated at all [pre/no-vax]) was 13.3 (8.7-23.2) months, while for pts in the "vaccinated" status (post-vax) was 7.9 (0-10.5) months.
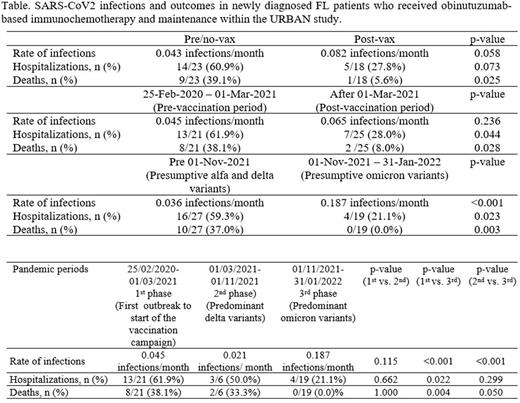
Overall, 48 COVID-19 infections were detected by swab in 45 pts, without any statistically significant correlation with age, ECOG PS, comorbidity, type of chemotherapy (CHOP, CVP or Bendamustine) or more recent tumor response.
Among the 48 COVID-19 infections, 23 occurred in 22 unvaccinated pts and 18 in 17 vaccinated pts (time relationship with vaccination was not available for 7 infections). Overall, a fatal outcome was reported in 10 cases (22.2% of infected pts), including 9 cases (39.1%) among unvaccinated pts, and a single case (5.6%) in those vaccinated (p<0.001). Hospitalization due to COVID-19 was required for 22 infections (45.8%), 14 (60.9%) in pre/no-vax pts, and 5 (27.8%) in post-vax ones (p=0.073). Interestingly, no differences in COVID-19 infection rates have been observed between pts who were receiving obinutuzumab maintenance during the pandemic and those who did not (p=1.000). The table summarizes COVID-19 infections and their outcomes according to vaccination status and by pandemic periods.
Conclusions. The URBAN retrospective/prospective study offered the unique opportunity to follow the impact of the evolving patterns of COVID-19 pandemic on a population of pts with advanced FL homogeneously treated with frontline obinutuzumab-based immunochemotherapy and maintenance. Specifically, we were able to gather the consequences of emerging SARS-CoV2 variants dynamically, before and after the availability of vaccines. From our results, the circulation of less aggressive COVID-19 variants and the increased vaccination coverage have led to better patients' outcomes, as observed in the healthy population, despite the B-cell lymphodepleting ability of obinutuzumab. Vaccination in FL pts enrolled in the study showed a protective effect in terms of rate of COVID-19-related risks for hospitalization and death. These findings should be interpreted with caution due to the variable concurrence of external factors, including different policies of contact restrictions, availability of diagnostic tests, and access to post-infection treatments across URBAN Centers during the analyzed periods. More data are currently being collected and will be presented at the conference.
Disclosures
Pinto:Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck Sharp and Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier Affaires Medicales: Honoraria; F. Hoffmann-La Roche AG, Incyte (Italy), Merck Sharp and Dohme, Servier Affaires Medicales: Honoraria; F. Hoffmann-La Roche AG, Merck Sharp and Dohme, Incyte (Italy): Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche AG: Honoraria, Membership on an entity's Board of Directors or advisory committees. Guardalben:Roche S.p.A.: Current Employment. Battista:Roche S.p.A.: Current Employment. Gazzoli:Roche S.p.A.: Current Employment. Murru:Janssen: Research Funding; Janssen, Abbvie, Astra Zeneca: Honoraria, Other: travel. Ferreri:Incyte: Membership on an entity's Board of Directors or advisory committees; Adienne: Speakers Bureau; Pfizer: Research Funding; Ospedale San Raffaele srl: Patents & Royalties: NGR-hTNAF/RCHOP in relapsed/refractory PCNSL; SNGR-hTNF in brain tumours; PletixaPharm: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Research Funding; Genmab: Research Funding; Amgen: Research Funding; Hutchison Medipharma: Research Funding; Pharmacyclics: Research Funding; Beigene: Research Funding; BMS: Research Funding. Ladetto:AbbVie: Honoraria; Acerta, Amgen: Honoraria; ADC Therapeutics: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; GSKI, Gentili, Sandoz: Honoraria; Gilead/Kite, Novartis: Honoraria; Roche, Eusapharma, Takeda, Regeneron: Honoraria; Regeneron, Incyte, Jazz: Honoraria; ADC Therapeutics: Honoraria, Research Funding; Jansenn: Honoraria, Research Funding. Zinzani:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; University of Bologna: Current Employment. Arcaini:Novartis: Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Celgene/Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Research Funding. Gritti:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; IQVIA: Membership on an entity's Board of Directors or advisory committees; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees; Italfarmaco: Membership on an entity's Board of Directors or advisory committees; Clinigen: Consultancy; Sandoz: Other: Support for attending meetings; Ideogen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy; Incyte: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.