Abstract
Background: Follicular lymphoma (FL) is an inherently immunosensitive cancer. While PD-1 blockade was associated with a low objective response rate (ORR) in FL, some durable responses were seen. Manipulation of other checkpoint pathways, like 4-1BB, have shown promising activity (Gopal, CCR 2020) and OX40 stimulation may enhance activity of other immunotherapies. We therefore conducted a multi-cohort phase 1b trial testing different combinations of immune checkpoint therapy targeting 4-1BB (utomilumab), OX-40 (PF-04518600), and PD-L1 (avelumab) all in combination with rituximab (R) among patients (pts) with relapsed/refractory (R/R) FL.
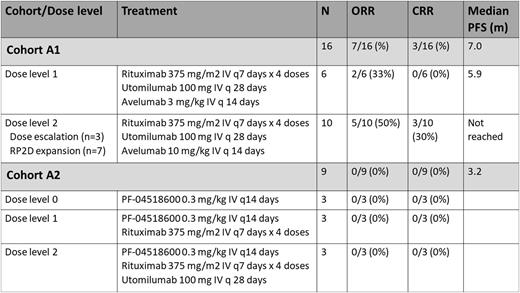
Methods: Adult pts with R/R grade 1-3a FL who had received ≥ 1 prior therapies (including a CD20 monoclonal antibody) were enrolled. All pts had measurable disease and required therapy based on modified GELF criteria. Pts in need of urgent cytoreductive therapy were excluded. Pts were enrolled onto 2 of 3 planned cohorts (A1 - R, utomilumab, avelumab; A2 - PF-04518600 +/- R, utomilumab). The trial was closed early when development of utomilumab and PF-04518600 was halted by Pfizer. Enrollment ended before dose expansion was completed for cohorts A1 and A2 and before enrollment began for a 3rd cohort (R, avelumab, PF-04518600). Each cohort began with a dose escalation stage (using a standard 3+3 design) followed by a dose expansion stage at the recommended phase 2 dose (RP2D) (15 pts). Treatment consisted of six 28-day cycles for both cohorts (see table for dose levels and dosing details). The co-primary endpoints were RP2D and complete response rate (CRR), assessed using 2014 Lugano criteria. Peripheral blood, stool samples, and tissue biopsies were collected for correlative studies.
Results: 24 pts were enrolled at 4 centers, including 1 pt who was initially treated on cohort A2 (with R + PF-04518600), ended treatment due to progression, and then enrolled on cohort A1. In total, 16 pts were treated on cohort A1 and 9 on cohort A2. The median age was 58 (42-76). Pts had received a median of 2 (1-9) prior therapies. 11 pts (46%) had progression within 24 months of initial therapy (POD24). At trial entry, 6 pts (25%) were R-refractory, defined as no response to last R-containing regimen or progression ≤6 months after last R dose.
The highest planned dose level (dose level 2) was selected as the RP2D for cohorts A1 and A2. There were no treatment discontinuations due to adverse events (AEs) and no treatment-related deaths. 1 dose-limiting toxicity (grade 3 gastric obstruction, unrelated to study treatment) was observed for a pt in cohort A1. In total, 19/25 (76%) pts had at least 1 treatment-related (tr)AE of any grade, including 7 (28%) pts with grade 3 trAEs. Typical immune-related AEs were not observed. The most common trAEs of any grade were fatigue (24%) and infusion reaction (20%). Grade 3 trAEs included neutropenia in 2 pts (8%), and hypertension, hypertriglyceridemia, hypophosphatemia, lymphopenia, COVID infection, and thromboembolic event in 1 pt each (4%).
In cohort A1, the ORR and CRR were 44% and 19%, respectively. Among 10 pts treated at the RP2D, the ORR and CRR were 50% and 30%, respectively. ORRs were observed in 2/5 POD24 pts and 1/4 R-refractory pts in cohort A1. In cohort A2, no responses were observed. After a median follow-up of 16 months, the median progression-free survivals in cohorts A1 and A2 were 7.0 and 3.2 months (m), respectively. The median duration of response in A1 was 9.7m. The 18m overall survivals in cohorts A1 and A2 were 81% and 100%, respectively.
We performed 16S ribosomal RNA sequencing of pre- and on-treatment stool samples from pts in cohort A1 and found a positive association between abundance of Akkermansia and ORR to study therapy (p<0.001). Other correlative studies including multiplex immunofluorescence and single-cell analyses (assessing serial biopsy samples) are ongoing.
Conclusions: We observed clinical activity with the combination of utomilumab, avelumab, and R, supporting a possible role for 4-1BB agonist therapy in FL. In contrast, our limited results do not support a role for OX40 targeting in this disease. Correlatives studies are ongoing which may shed further light on predictors of response. Interestingly, we found an association between Akkermansia and response, which has been reported in checkpoint blockade studies in solid tumors and supports the hypothesis that the microbiome impacts response to immunotherapies in lymphomas.
Disclosures
Merryman:Genentech/Roche: Research Funding; Epizyme: Consultancy; Merck & Co., Kenilworth, NJ: Research Funding; Pfizer: Research Funding; Bristol Myers Squib: Consultancy, Research Funding; Abbvie: Consultancy; Intellia: Consultancy; Adaptive Biotechnologies: Consultancy; Genmab: Consultancy, Research Funding. Brown:BeiGene, Gilead, Loxo/Lilly, MEI Pharma, SecuraBio, Sun, TG Therapeutics: Research Funding; Abbvie, Acerta/Astra-Zeneca, BeiGene, Bristol-Myers Squibb/Juno/Celgene, Catapult, Eli Lilly, Genentech/Roche, Hutchmed, iOnctura, Janssen, MEI Pharma, Pharmacyclics: Consultancy. Crombie:Incyte: Consultancy; Roche: Research Funding; Merck: Research Funding; Karyopharm: Consultancy; Abbvie: Research Funding; Kite: Consultancy; Bayer: Research Funding. Davids:Eli Lilly and Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Research Funding; Ascentage Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Research to Practice: Honoraria; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ono Pharmaceuticals: Consultancy; Merck: Consultancy; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees. LaCasce:Research to Practice: Consultancy; Seattle Genetics: Consultancy. Isufi:Kite: Speakers Bureau; Bayer: Honoraria; Epizyme: Membership on an entity's Board of Directors or advisory committees; BEAM Therapeutics: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees. Kline:Karyopharm, Kite/Gilead, Merck, MorphoSys, Seagen, Verastem: Consultancy; iTeos, Merck, Verastem: Research Funding. Cohen:Lilly Oncology/Eli Lilly: Consultancy, Research Funding; Aptitude Health: Consultancy; Janssen: Consultancy; BeiGene: Consultancy, Research Funding; Takeda: Research Funding; Kite Pharma/Gilead: Consultancy; HutchMed: Consultancy, Research Funding; Astrazeneca: Consultancy, Research Funding; Novartis: Research Funding; Genentech: Research Funding; BMS/Celgene: Research Funding. Mehta-Shah:Verastem: Research Funding; Secura Bio: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa Hakko Kirin Co., Ltd.: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genetech/Roch: Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Research Funding; Corvus Pharmaceuticals: Research Funding; Celgene: Research Funding; Bristol Myers-Squibb: Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bartlett:Autolus, Bristol-Meyers Squibb, Celgene, Forty Seven, Janssen, Kite Pharma, Merck, Millennium, Pharmacyclics: Research Funding; Washington University School of Medicine: Current Employment; ADC Therapeutics, Roche/Genentech, Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Mei:CTI: Honoraria; Morphosys: Research Funding, Speakers Bureau; Novartis: Consultancy; EUSA: Honoraria; Celgene: Research Funding; Beigene: Research Funding; Incyte: Research Funding. Armand:IGM: Research Funding; Adaptive: Research Funding; Kite: Research Funding; Xencor: Consultancy; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy; Epizyme: Consultancy; Regeneron: Consultancy; Enterome: Consultancy; C4: Consultancy; GenMab: Consultancy; Tessa Therapeutics: Consultancy; ADC Therapeutics: Consultancy; BMS: Consultancy, Research Funding; Merck: Consultancy, Honoraria, Research Funding. Jacobson:Instil Bio: Consultancy; Ipsen: Consultancy; Epizyme: Consultancy; Bluebird Bio: Consultancy; BMS/Celgene: Consultancy; Novartis: Consultancy; Pfizer: Research Funding; Abintus Bio: Consultancy; Morphosys: Consultancy; Miltenyi: Consultancy; Caribou Bio: Consultancy; Kite/Gilead: Consultancy, Research Funding; ImmPACT Bio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.