Abstract
Background: Systemic chronic active Epstein-Barr virus disease (sCAEBV) is a rare EBV-positive T- or NK-lymphoproliferative disease. Although the only curative treatment for sCAEBV available now is allogeneic hematopoietic stem cell transplantation (allo-HSCT), the presence of disease activity, persistent inflammation, at the time of allo-HSCT is associated with poor outcomes (Am J Hem.2022, 97, p780). STAT3 is constitutively activated contributing to cytokine production and cell survival of EBV-infected cells in sCAEBV (Oncotarget. 2019, 9, p31077). Thus, STAT3 inhibition by ruxolitinib, a JAK1/2 inhibitor, may be effective against disease activity of sCAEBV.
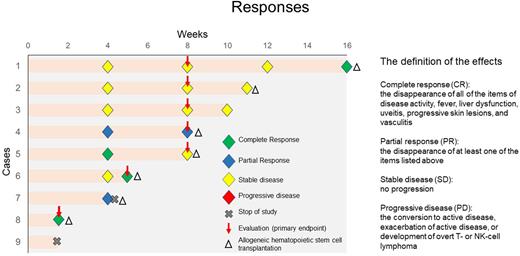
Methods: We conducted an investigator-initiated trial of open-label, phase 2, prospective and multicenter to assess the efficacy and safety of ruxolitinib in sCAEBV patients with disease activity. The diagnostic criteria were as follows: elevated EBV-DNA load in the peripheral blood (PB) (>102.5 copies/µg DNA); EBV infected T or NK cells detected in affected tissues or PB; systemic inflammatory symptoms persisting for more than three months; exclusion of other possible diagnoses, such as immune deficiencies, autoimmune diseases, or lymphoma (Blood adv. 2021, 4, p2918). sCAEBV patients ≥13 y.o. with fever (≥ 37.5 ºC), and/or liver dysfunction (ALT ≥ 2.0 × ULN) were enrolled. Anti-VCA-IgM-positive patients were excluded. The initiation dose of ruxolitinib was 5-20 mg twice a day determined by the platelet count in PB. The treatment effects were classified as complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD). CR was defined as the disappearance of all the elements of disease activity such as fever, liver dysfunction, uveitis, progressive skin lesions, and vasculitis. PR was defined as the disappearance of at least one of the elements listed above. PD was defined as the conversion to active disease, exacerbation of active disease, or the development of overt T- or NK-cell lymphoma. SD was defined as presenting no progression. Ruxolitinib was administered for eight weeks. If CR was observed and allo-HSCT was scheduled, ruxolitinib was terminated early even if these events had occurred within less than eight weeks after initiating investigational drug administration. The primary endpoint of efficacy assessments was CR rate at eight weeks after the initiation of drug administration or at the time of early termination. Safety was assessed based on the frequency of adverse events defined by Common Terminology Criteria for Adverse Events, version 4.0.
Results: Nine patients were enrolled between January 2019 and August 2021; five males and four females aged 13-64 y.o.; median age of 40 y.o. The types of EBV-infected cells were as follows: four were CD4, one was CD8, and four were CD56. Seven patients completed the study. Five among them received ruxolitinib for eight weeks and were treated as outpatients. Two patients fulfilled the criteria of early termination and each was treated for 35 and 12 days. The responses are shown in the figure. CR rate and OR rate after eight weeks or at early termination, the primary and the secondary endpoints were 28.6% (2/7) and 42.9% (3/7), respectively. No one showed progressive disease. One patient developed a grade 4 adverse event of oral bleeding during the treatment and resulted in discontinuing the administration of ruxolitinib on day 13. Seven patients received allo-HSCT. Five patients achieved undetectable level of EBV-DNA PB after allo-HSCT.
Conclusion: The results indicate that ruxolitinib has demonstrated encouraging effects and is a potent therapeutic reagent against sCAEBV with active disease. Further analysis shall complete its assessment.
Disclosures
Yamamoto:NIPPON SINYAKU CO., LTD: Honoraria; Novartis Pharma K.K.: Honoraria; ONO PHARMACEUTICAL CO.: Honoraria; Otsuka Pharmaceutical: Honoraria; Abbvie GK: Honoraria; Takeda Pharmaceuticals: Honoraria; Sanofi: Honoraria; Meiji Seika Pharma Co., Ltd: Honoraria; Bristol-Myers Squibb Company: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Eisai Co., Ltd.: Honoraria; Symbio Pharmaceuticals: Honoraria. Arai:Asahi Kasei Pharma Corporation: Research Funding; Shionogi & Co., Ltd.: Research Funding; Takeda Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Novartis Pharma K.K.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Research Funding; Nippon Shinyaku Co., Ltd.: Honoraria, Research Funding; Ono Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Kyowa Kirin Co., Ltd.: Honoraria, Research Funding; Abbott Japan LLC: Honoraria; Eisai Co., Ltd.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Bristol-Myers Squibb K.K.: Honoraria; AbbVie GK: Honoraria; Sanofi K.K.: Honoraria; Pfizer Japan Inc.: Honoraria; Astellas Pharma Inc.: Honoraria.
OffLabel Disclosure:
Drug: Ruxolitinib Purpose: to investigate the effects of ruxolitinib on the disease activity of systemic chronic active Epstein-Barr virus disease (sCAEBV), a rare and intractable EBV-positive T- or NK-lymphoproliferative disease.
Author notes
Asterisk with author names denotes non-ASH members.