Abstract
Introduction: As outcomes of patients with acute myeloid leukemia (AML) have improved, the fraction of patients surviving long-term is increasing. Information on somatic and psycho-social health consequences of AML and its treatment is sparse. The aim of our study was to perform a multi- dimensional analysis of health outcomes in AML long-term survivors (AML-LTS). This report focuses on secondary neoplasms (SN) after AML. Data on psychosocial outcomes and clonal hematopoiesis are presented by Telzerow et al., Görlich et al. and Krauss et al.
Methods: We conducted a cross-sectional study including AML survivors who had been enrolled in clinical trials or the patient registry of the AML-CG study group, and were alive ≥5 years after initial diagnosis. Data concerning somatic health status were collected through patient questionnaires, assessments by the patients' physicians, and medical and laboratory reports. (DRKS00023991)
Results: Data on somatic health status is available for 355 AML-LTS, 58% female, aged 28 to 93 years [y] (median 60y), 5 to 19y after initial diagnosis (median, 11.6y). Sixteen percent were initially diagnosed with secondary AML (sAML) or therapy-related AML (tAML) and 38% were treated with chemotherapy only while 62% had undergone allogeneic stem cell transplantation (alloHSCT).
Fifty-five AML-LTS (15.5%) had developed SN after AML, that manifested 3 months to 18y after initial diagnosis (median, 12.4y), at age 31 to 83y (median 59y). Forty-two percent of AML-LTS with SN were female, 66% had undergone alloHSCT and 24% were initially diagnosed with sAML or tAML. At the time of study participation, 96% percent of all AML-LTS and 90 % of those diagnosed with SN fulfilled CR criteria (neutrophils >1G/L and thrombocytes >100G/L).
Most common tumor types were non-melanoma skin cancers (30%), breast cancer (11%, all female, 25% of female AML-LTS with SN), head and neck cancers (7%) and, in 5% each, cancers of the esophagus, lung, bladder and prostate (9.4% of male AML-LTS with SN).
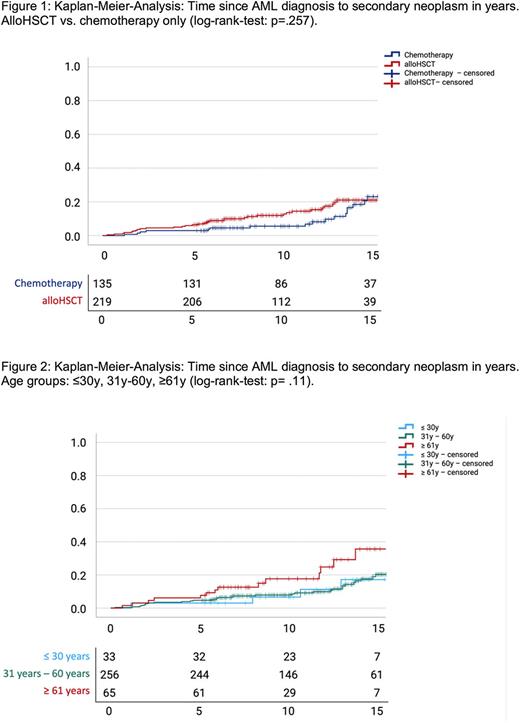
Kaplan-Meier-Analyses show a 20%-risk of AML-LTS to develop SN 15 years after initial diagnosis, with no significant difference between patients treated by alloHSCT or chemotherapy only (log-rank p=.26; Fig. 1). However, 10y-risk was 5.6% and 15y-risk was 18.5% for AML-LTS treated with chemotherapy only, whereas for AML-LTS treated with alloHSCT 10y-risk was 12% and 15y-risk was 21%.
AML-LTS >60y at the time of AML diagnosis had a significantly higher risk to develop secondary cancer, with a 10y-risk of 17.7% compared to 7.9% for AML-LTS aged 30-60y and 6.6% aged <30y at initial diagnosis (Fig. 2).
Using Cox regression models, we analyzed the influence of age at AML diagnosis, sex, smoking status, sAML/tAML vs. de novo-AML, AML relapse (as time-dependent covariable) and treatment with alloHSCT vs. chemotherapy only on the risk of developing secondary malignancies after AML. Only older age at initial diagnosis (OR=1.03 per year, 95% CI: 1.01 - 1.05, p=.01) and male sex (OR=1.93, 95%-CI: 1.12 - 3.37, p=.02), but none of the diagnosis- and treatment-related factors significantly associated with higher risks for secondary malignancies. However, AML-LTS diagnosed with sAML/tAML tended to develop SN earlier after initial diagnosis than those diagnosed with de novo AML. Ten-years-risk was 9% and 15y-risk was 20% for AML-LTS diagnosed with de-novo-AML, whereas for AML-LTS diagnosed with sAML/tAML 10y-risk was 14% and 15y-risk was 23% (log-rank p=.08).
Conclusion: Our study includes a large cohort of AML-LTS representing a wide age range, a long follow-up period of 5 to nearly 20 years, and heterogeneous therapy regimens (chemotherapy + autoHSCT vs. alloHSCT), close to real-life clinical settings.
We found that the 15y-risk to develop a SN after AML is ~20%, with men having a 2-fold higher risk, and older age at the time of diagnosis being a negative predictor. We did not identify diagnosis- or treatment-related factors associated with increased risks for SN, although SN tended to occur earlier in patients who received alloHSCT compared to those treated with chemo only. We hope that our results may guide future recommendations for risk-adapted follow-up of AML-LTS.
Disclosures
Krug:AbbVie: Honoraria; BMS: Honoraria; , Leo Pharma,: Honoraria; , Sanofi: Honoraria. Metzeler:Novartis: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy; Pfizer: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; AbbVie: Honoraria; Curis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.