Abstract
Introduction: Daratumumab (DARA) is approved across lines of therapy for multiple myeloma. In the primary analysis of the randomized phase 2 GRIFFIN trial (NCT02874742) (median follow-up, 13.5 mo), addition of DARA to RVd improved the stringent complete response (sCR) rate by end of post-autologous stem cell transplant (ASCT) consolidation (D-RVd, 42.4% vs RVd, 32.0%; odds ratio [OR], 1.57; 95% CI, 0.87-2.82; 1-sided P = 0.068, which met the pre-specified 1-sided α of 0.1) (Voorhees PM, et al. Blood. 2020). Here, we present an end-of-study post hoc analysis of clinically relevant subgroups in GRIFFIN (≥65 years; International Staging System [ISS] stage III disease; high cytogenetic risk [defined as ≥1 of the following: del17p, t(4;14), or t(14;16)]; revised high cytogenetic risk [defined as ≥1 of the following high-risk chromosomal abnormalities (HRCA): del17p, t(4;14), t(14;16), t(14;20) or gain/amp1q (≥3 copies of chromosome 1q21)]; gain/amp1q; 1 HRCA (per the revised definition); gain/amp1q plus 1 HRCA; and ≥2 HRCA). The final analysis of GRIFFIN (median follow-up, 49.6 mo) occurred after all pts completed ≥1 year of long-term follow-up after completion of study treatment, discontinued, or withdrew.
Methods: In GRIFFIN, pts with transplant-eligible NDMM were randomized 1:1 to D-RVd or RVd. All pts received 4 D-RVd/RVd induction cycles, ASCT, 2 D-RVd/RVd consolidation cycles, and maintenance with lenalidomide (R) ± DARA for 24 months. All pts received 21-day cycles of R (25 mg PO on Days [D] 1-14), bortezomib (1.3 mg/m2 SC on D1, 4, 8, 11), and dexamethasone (40 mg PO QW) ± DARA (16 mg/kg IV QW in Cycles 1-4 and D1 in Cycles 5-6). During maintenance (Cycles 7-32; 28-day cycles), pts received R (10 mg PO on Days 1-21; if tolerated, 15 mg in Cycles 10+) ± DARA (16 mg/kg IV Q8W/Q4W or 1800 mg SC Q4W per protocol amendments). The primary endpoint was the sCR rate by end of consolidation.
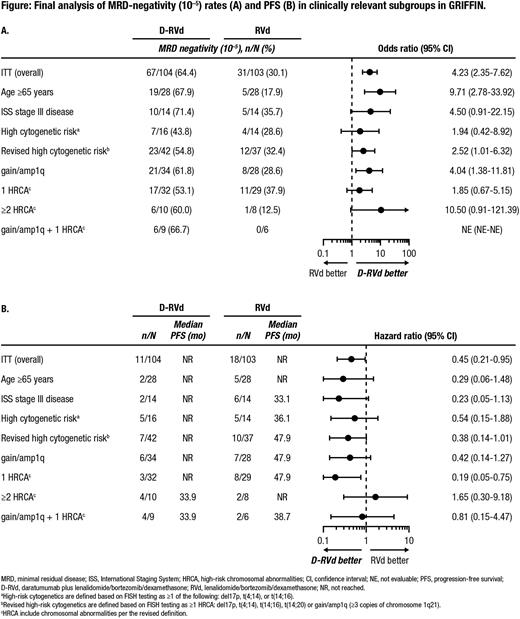
Results: 207 pts were randomized (D-RVd, n = 104; RVd, n = 103); each subgroup had a similar number of pts per arm: ≥65 years (n = 28; n = 28), ISS stage III disease (n = 14; n = 14), high cytogenetic risk (n = 16; n = 14), revised high cytogenetic risk (n = 42; n = 37), gain/amp1q (n = 34; n = 28), 1 HRCA (n = 32; n = 29), gain/amp1q plus 1 HRCA (n = 9; n = 6), and ≥2 HRCA (n = 10; n = 8). Outcomes for pts with baseline extramedullary plasmacytomas (D-RVd, n = 1; RVd, n = 2) were explored but not included due to small pt numbers. Among response-evaluable pts, the rate of sCR at the end of maintenance therapy was numerically higher for D-RVd versus RVd among pts with age ≥65 years (63.0% vs 40.7%; OR, 2.47; 95% CI, 0.83-7.39), high cytogenetic risk (50.0% vs 38.5%; OR, 1.60; 95% CI, 0.36-7.07), gain/amp1q plus 1 HRCA (55.6% vs 33.3%; OR, 2.50; 95% CI; 0.29-21.40), and ≥2 HRCA (50.0% vs 37.5%; OR, 1.67; 95% CI, 0.25-11.07), but similar for D-RVd and RVd pts with ISS stage III disease (64.3% vs 61.5%; OR, 1.13; 95% CI, 0.24-5.37), revised high cytogenetic risk (56.1% vs 55.6%; OR, 1.02; 95% CI, 0.42-2.52), gain/amp1q (57.6% vs 57.1%; OR, 1.02; 95% CI, 0.37-2.82), and 1 HRCA (58.1% vs 60.7%; OR, 0.90; 95% CI, 0.32-2.54). MRD-negativity (10-5) rates at the end of maintenance favored D-RVd over RVd across all subgroups (Figure A). At 49.6 months of median follow-up, PFS rates among subgroups favored D-RVd over RVd except for pts with ≥2 HRCA (Figure B).
In pts ≥65 years, grade 3/4 treatment-emergent adverse events (TEAEs) occurred in 88.9% of D-RVd and 77.8% of RVd pts; the most common grade 3/4 TEAEs (≥20%) were neutropenia (D-RVd, 37.0%; RVd, 29.6%) and lymphopenia (25.9%; 11.1%). TEAEs led to study treatment discontinuation in 37.0% of D-RVd and 25.9% of RVd pts. In pts ≥65 years, death as outcome of a TEAE occurred in 1 D-RVd pt (pneumonia; unrelated to study treatment).
Conclusions: In this final analysis of GRIFFIN by clinically relevant subgroups, addition of DARA to RVd induction/consolidation and R maintenance, with ASCT, was associated with higher MRD-negativity (10-5) rates for all subgroups and PFS estimates favored all high-risk groups except pts with ≥2 HRCA. Among pts ≥65 years, the rates of grade 3/4 TEAEs and TEAEs leading to study treatment discontinuation were slightly higher for the D-RVd group, although only 1 pt died due to a TEAE unrelated to study treatment. Results of this subgroup analysis support the use of DARA in transplant-eligible pts with NDMM among both clinically and cytogenetic high-risk subgroups, although larger studies are needed, especially in pts with ≥2 HRCA.
Disclosures
Kaufman:AbbVie: Other: Member of steering committee; AbbVie, Genentech, and Bristol Myers Squibb: Consultancy; Incyte: Other: Member of data safety monitoring committee . Laubach:Lignancies: Honoraria. Sborov:GlaxoSmithKline, Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Skyline Dx, Janssen, AbbVie, Sanofi: Consultancy; BMS: Consultancy. Reeves:Incyte, BMS, PharmaEssentia, CTI Biopharma: Honoraria; Hemostasis & Thrombosis Research Society Mentored Research Award sponsored by CSL Behring: Research Funding. Rodriguez:Janssen, BMS, Takeda, AbbVie, karyopharm, Artiva: Consultancy, Speakers Bureau. Silbermann:Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Costa:Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria; Genentech: Research Funding; AbbVie: Research Funding; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Anderson:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Prothena: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Shah:GSK, Amgen, Indapta Therapeutics, Sanofi, CareDx, Kite, Karyopharm, Oncopeptides,: Consultancy; AstraZeneca: Current Employment, Current equity holder in publicly-traded company; Aztra Zeneca: Current Employment, Other: stock ownership; Celgene/BMS, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, Nektar, Precision Biosciences: Research Funding. Bumma:Sanofi, Genzyme: Other: Ad Board, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Ad Board; Amgen: Consultancy, Speakers Bureau. Holstein:Genentech: Consultancy; Janssen: Consultancy, Research Funding; Secura Bio: Consultancy; Oncopeptides: Consultancy, Research Funding; BMS/Celgene: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Sanofi: Consultancy. Costello:BMS, Takeda, Janssen, Pfizer: Honoraria, Research Funding. Jakubowiak:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Wildes:Janssen: Consultancy; Sanofi: Consultancy; Seattle Genetics: Consultancy; Carevive: Consultancy. Orlowski:Abbvie, BioTheryX, Inc., Bristol-Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Inc., Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda Pharmaceutic: Honoraria, Membership on an entity's Board of Directors or advisory committees; CARsgen Therapeutics, Celgene/Bristol Myers Squibb, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; Asylia Therapeutics, Inc., BioTheryX, Inc., Heidelberg Pharma, Inc.: Research Funding; Asylia Therapeutics, Inc.: Current equity holder in private company. Shain:Janssen,BMS: Other: PI of clinical trials; AbbVie, Karyopharm: Research Funding; GSK, Janssen and BMS and speaker's bureau for GSK, BMS, Sanofi, Karyopharm, Takeda, Janssen, Adaptive and Amgen: Other: Advisory Committee; Bristol Myers Squibb (BMS), Janssen, GlaxoSmithKline (GSK), Adaptive, Sanofi, and Takeda, and Amgen: Honoraria. Cowan:Secura bio: Consultancy; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; EUSA: Consultancy; Allogene: Consultancy; AbbVie: Consultancy, Research Funding; Nektar: Research Funding; BMS: Consultancy, Research Funding; Sanofi-Aventis: Research Funding; Harpoon: Research Funding; Janssen: Consultancy, Research Funding. Pei:Janssen: Current Employment, Current equity holder in publicly-traded company. Cortoos:Janssen: Current Employment, Current equity holder in publicly-traded company. Patel:Companies of Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Lin:Janssen: Current Employment, Current holder of stock options in a privately-held company. Richardson:Takeda, Abbvie, GSK, and Celgene: Consultancy; Regeneron: Consultancy; GlaxoSmithKline: Consultancy; AstraZeneca: Consultancy; Secura Bio: Consultancy; Sanofi: Consultancy; Karyopharm: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; Protocol Intelligence: Consultancy; Oncopeptides: Consultancy, Research Funding; Takeda: Research Funding; Takeda, Celgene, and GSK: Honoraria; Takeda and GSK: Other: Travel expenses from Takeda and GSK. Usmani:Amgen, BMS, Janssen, Sanofi: Speakers Bureau; Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio: Consultancy; Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda: Research Funding. Voorhees:Abbvie, Amgen, BMS, GSK, Karyopharm, Novartis, Oncopeptides, Pfizer, Sanofi, SecuraBio: Consultancy, Honoraria.
OffLabel Disclosure:
The specific regimen combination is not yet approved, but individual components are.
Author notes
Asterisk with author names denotes non-ASH members.