Abstract
Background: For decades, CD71+ erythroid cells (CECs) were considered to be mere progenitors and precursors of oxygen-transporting erythrocytes. However, increasingly more observations indicate that they are also crucial regulators of the immune response in various stages of life. CECs use various mechanisms to regulate immune response in different niches, including arginase-2, reactive oxygen species, or checkpoint molecules. However, factors regulating immunomodulatory properties of CECs remain largely unknown, making it difficult to develop effective therapies exploiting immunomodulatory functions of these cells. A common condition under which CECs potently suppress the immune response in fetal life, pregnancy, or advanced cancer is hypoxia. Thus, we hypothesized that hypoxia regulates the immunoregulatory functions of CECs.
Methods: Murine CECs were isolated from the spleens of anemic C57BL/6 mice using immunomagnetic selection with an anti-CD71 antibodies. Murine T-cells were isolated from the spleens of control C57BL/6 mice using negative immunomagnetic selection. Three model erythroid cell lines HEL.92.1.7, TF-1, and K562 were cultured according to the ATCC protocols. Cells were transduced with three different lentiviral vectors encoding shRNA targeting HIF-1α mRNA as well as non-targeting scramble controls. Human CECs were expanded from peripheral blood mononuclear cells (PBMC) obtained from healthy donors according to Heshusius et al. (2019). To determine the immunoregulatory properties of CECs, a T-cell proliferation assay and cytokine production assay were used. The Biospherix X-Vivo System was used to evaluate the impact of different oxygen concentrations on the immunoregulatory properties of CECs. In some experiments, HIF-1α was stabilized in normoxia using hypoxia-mimicking agents, cobalt chloride (CoCl2, a PHD inhibitor), and IOX4 (a competitive and selective HIF prolyl hydroxylase 2 (PHD2) inhibitor). Flow cytometry analyses were performed using BD LSRFortessa™ X-20, BD FACSCanto™ II operated by BD FACSDiva Software. For data analysis, Flow Jo v10.6.1 software (TreeStar) was used. The expression of mRNA was determined using the real-time qPCR method using the GoScript™ Reverse Transcriptase system (Promega) and SYBR Green (ThermoFisher).
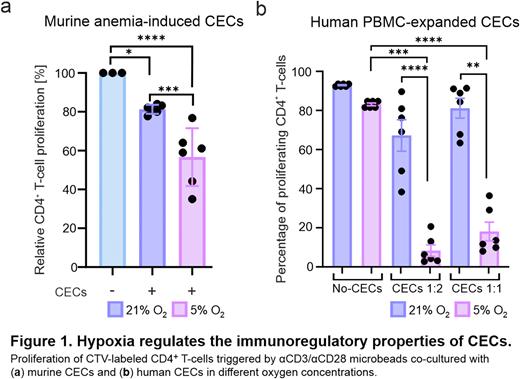
Results: We observed that induction of non-hemolytic anemia that triggers expansion of CECs resulted in an increased levels of HIF-1α in CECs in the murine spleen. Next, to determine the effects of hypoxia on immunoregulatory properties of murine CECs, they were isolated from the spleens of anemic mice and co-cultured with murine T-cells triggered to proliferate by anti-CD3/anti-CD28 microbeads in 1%, 5% (hypoxia) and 21% (normoxia) oxygen concentration. We found that suppression of T-cell proliferation by CECs is potentiated in hypoxic conditions. To evaluate the effects of hypoxia on human CECs, we used CECs expanded from PBMC (PBMC-CECs) collected from healthy donors. We found that hypoxia potentiated PBMC-CECs-driven suppression of proliferation as well as production of IFN-γ by T-cells. Moreover, immunoregulatory properties of human model erythroid cell lines HEL.92.1.7, TF-1, and K562 were regulated by oxygen concentration and potentiated in hypoxic conditions. Hypoxia, as well as stabilization of HIF-1α with hypoxia-mimicking agents, upregulated the expression of immunomodulatory molecules in both murine and human CECs. To determine the role of HIF-1α, a key hypoxia-inducible transcription factor, in the regulation of immunoregulatory properties of CECs, we transduced erythroid cell lines with lentiviral vectors encoding shRNA targeting HIF-1α. Notably, downregulation of HIF-1α in erythroid cell lines resulted in the decreased expression of immunomodulatory molecules and attenuation of their suppressive effects on T-cell proliferation.
Conclusions: Our results suggest that the immunoregulatory properties of CECs are regulated by the oxygen concentration and are potentiated in hypoxic conditions. More studies are performed to dissect the molecular mechanisms of these effects.
Disclosures
Basak:Amgen: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Saventic Health: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Human Biome Institute: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.