Abstract
CD19-directed chimeric antigen receptor (CART19) cells have emerged as a potentially curative immunotherapy in a subset of patients with hematological malignancies. While initial responses are impressive, the majority of responsive patients relapse within a year. Recent studies suggest that CART cells are susceptible to states of dysfunction.
We aimed to study the development of T cell senescence in CART cells using similar constructs to the FDA-approved therapies, CART19-BBζ and CART19-28ζ, to determine the impact of co-stimulatory domains on CART cell senescence. We developed an in vitro model for repeated CART cell activation followed by rest to study the development of senescence and its impact on effector T cell functions. We examined CART cell immunophenotype, cell cycle regulators, and transcriptomic profile on days 0 (T cell), D8 (standard CART cell), 15 (after one activation cycle) and 22 (after two activation cycles).
Our immunophenotypic analyses suggest differential expression in inhibitory receptors between CART19-BBζ and CART19-28ζ following CART cell stimulation and rest. TIM-3 is upregulated in both CART19-BBζ (D0 vs D8 and D15, p=0.0289 and p=0.0090, respectively) and CART19-28ζ (D0 vs D22 p=0.0394) while CTLA-4 is slightly but significantly upregulated in only CART19-28ζ (D0 vs D8, D15, and D22; p=0.0002, p=0.0096, and p=0.0305, respectively). LAG-3 and PD-1 expression levels did not increase in either CART19-BBζ or CART19-28ζ.
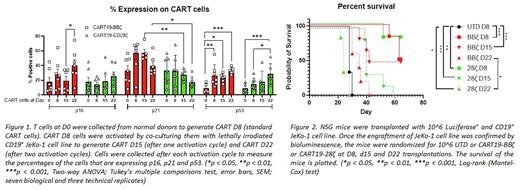
Since increased TIM-3 expression is associated with T cell senescence, we further investigated p16, p21 and p53 by flow cytometry as the levels of these proteins are frequently increased in senescent cells. p16 levels from D15 to D22 (p= 0.0165) and p53 levels from D0 to D8, D15 and D22 are increased in CART19-BBζ (p= 0.0071, p=0.0281, and p= 0.0002, respectively) but not in CART19-28ζ. p53 expression in CART19-28ζ increased at a later time point (D0 vs D8 and D8 vs D22, p=0.4951, p=0.0257, respectively). The expression of p21 did not change upon serial activation for either CART19-BBζ or CART19-28ζ; however, p21 levels were higher in CART19-BBζ at D15 and D22 (p= 0.0056, p= 0.0113, respectively) compared to CART19-28ζ (Figure 1). CD28 levels decreased from D8 to D15 and D22 in CART19-BBζ (p= 0.0327 and p= 0.0406, respectively) while CD28 levels did not change in CART19-28ζ. Changes in the level of these markers suggest a more prominent senescent phenotype in CART19-BBζ compared to CART19-28ζ.
Then, we evaluated the transcriptomic signature of cells acquiring a senescent phenotype by performing RNA sequencing of CART19 cells at D0, D8, D15 and D22 in our in vitro repeated activation model. GSEA indicated enrichment of gene sets related to senescent T cells such as p53, DNA damage response, cell cycle checkpoint, glycolysis, and oxidative phosphorylation in CART19-BBζ D15 compared to CART19-BBζ D8 or CART19-28ζ D15. We used a novel gene set, senMayo, developed at Mayo Clinic to define senescing cell or tissue populations. This gene set is also significantly enriched in CART19-BBζ D15 compared to D0 or CART19-28ζ D15 (p=0.0053, p=0.16, respectively) suggesting a senescent fate in CART19-BBζ cells.
Next, to study the antitumor activity of activated/rested CART19 in vivo, we generated xenograft models through the engraftment of NOD-SCID-γ-/- (NSG) mice with the luciferase+ CD19+ cell line JeKo-1. Engrafted mice were randomized based on bioluminescence to treatment with UTD (untransduced T cell control), D8, D15 and D22 CART19-BBζ or CART19-28ζ (n≥5 mice/group). Mice treated with CART19-BBζ D8, CART19-BBζ D15, CART19-28ζ D8, and CART19-28ζ D15 had significantly less tumor burden than UTD mice (p= 0.0005, p= 0.0013, p= 0.0004 and p= 0.0012, respectively). However, mice treated with CART19-BBζ D22 or CART19-28ζ D22 CART cells had similar tumor burden to UTD mice. Interestingly, CART19-BBζ D22 mice had a significantly lower tumor burden compared to CART19-28ζ D22 mice (p= 0.0140). The survival of the mice correlated well with the tumor burden (Figure 2). Collectively, our results indicate that CART19-BBζ cells are more susceptible to developing a senescent phenotype compared to CART19-28ζ as indicated by persistent expression of TIM-3, elevated cell cycle regulators, and a predominant transcriptional signature of senescence. Better understanding of different T cell fates in CART failure will facilitate development of strategies to improve CART efficacy.
Disclosures
Sakemura:Humanigen: Patents & Royalties. Cox:Humanigen: Patents & Royalties. Kenderian:Mettaforge: Patents & Royalties; Lentigen: Research Funding; MustangBio: Patents & Royalties; Humanigen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau; Tolero: Research Funding; LEAH Labs: Current holder of stock options in a privately-held company, Research Funding; Life Engine: Current holder of stock options in a privately-held company; Viracta/Sunesis: Research Funding; Morphosys: Research Funding; Juno/BMS: Consultancy, Research Funding, Speakers Bureau; Kite/Gilead: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau. Kay:Rigel: Other: Data Safety Monitoring Committee; Morpho-sys: Other: Data Safety Monitoring Committee; Dren Bio: Other: Data Safety Monitoring Committee; BMS: Other: Data Safety Monitoring Committee, Research Funding; Targeted Oncology: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitoring Committee; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Cytomx Therapy: Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitoring Committee; Behring: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitoring Committee; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Other: Data Safety Monitoring Committee, Research Funding; Genentech: Research Funding; MEI Pharma: Research Funding; Sunesis: Research Funding; TG Therapeutics: Research Funding; Tolero Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.