Abstract
Despite of the continuous improvements in the treatment for acute myeloid leukemia (AML), therapy resistance and disease relapse have been persistent and attributed to leukemia stem cells (LSCs). Recent single cell RNA sequencing (Galen et al, Bernstein 2019) and deconvoluted bulk RNA sequencing analysis (Zeng et al, Dick 2022) show hierarchical cellular organization in individual AML patient specimens, with LSCs, and quiescent LSCs in particular, at its apex. However, the mechanisms controlling LSCs and their quiescence remain elusive, hindering the development of effective targeted therapies. Here, using human patient AML specimens, we show that activator protein 1 (AP-1) and E26 transformation-specific (ETS) family transcription factors (TFs) coordinately regulate LSC quiescence and disease persistence after chemotherapy.
A chemical label tracing technique using carboxyfluorescein succinimidyl ester (CFSE) has emerged as a powerful tool to identify quiescent LSCs, and using this technique, we and others have demonstrated that CFSE-label retaining quiescent LSCs isolated from patient leukemia cells exhibit potent leukemia initiating property, resistance to conventional chemotherapy, and epigenetic plasticity (Takao et al, Kentsis 2018 ASH, Ebinger et al, Jeremias 2016 and 2020). Here, we combined this method with functional genomic approaches to elucidate the mechanisms controlling quiescent LSCs and identify novel therapeutic targets to overcome chemotherapy resistance.
First, to identify the candidate genes for LSC quiescence regulators, we performed gene expression and chromatin accessibility profiling of label-retaining quiescent LSCs isolated from human patient leukemia cell-engrafted NSG mice in diverse subtypes of AMLs using mRNA sequencing (RNA-seq) and assay for transposase-accessible chromatin with high-throughput sequencing (ATAC-seq). We identified that several transcription factors important for normal hematopoiesis, which included AP-1 TFs, such as JUN and JUNB, and ETS TFs, such as ETS1 and FLI1, were differentially expressed in quiescent LSCs. Chromatin accessibility profiling further revealed the predominant occupancy of AP-1 and ETS TF family binding motifs in differentially accessible chromatin loci in quiescent LSCs. Importantly, gene expression signatures detected in quiescent LSCs showed significant similarity to gene expression profiles of residual leukemia cells observed in chemotherapy-treated AML patients (GSE116256, Galen et al. 2019), supporting the clinical relevance of our models.
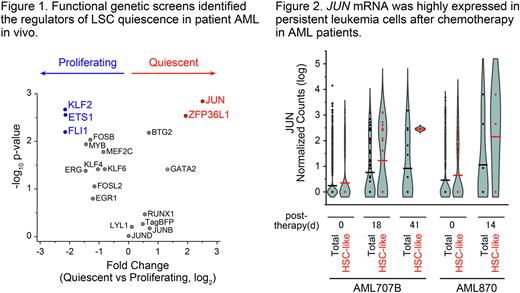
Next, to develop a functional screen system to identify the regulators of quiescent LSCs in human patient AML-bearing mice, we designed a lentiviral vector encoding mCherry fluorescent protein and doxycycline-inducible cDNAs marked by specific barcode sequences. cDNA library screening using this lentiviral vector identified several TFs as regulators for AML LSC quiescence, which included JUN as a positive regulator, and ETS1 and FLI1 as negative regulators (Figure 1).
Finally, given the evidence of transcriptional activity of JUN to regulate LSC quiescence, we hypothesized that JUN activity may be also associated with chemotherapy resistance and disease persistence in patients. To investigate this, we analyzed JUN expression in individual AML cells in diverse patients before and after chemotherapy treatment (GSE116256, Galen et al. 2019). We found JUN to be highly expressed in persistent AML cells upon induction chemotherapy, especially in leukemia cells with features of hematopoietic stem cells (HSC-like), while ETS1 and FLI1 expression was not (Figure 2). These results argue that JUN activity may be required for chemotherapy resistance, LSC persistence, and ultimate AML relapse. In all, human AML LSC quiescence and therapy response can be dynamically regulated by a distinct transcription factor network.
This work defines molecular mechanisms controlling AML LSC quiescence. We anticipate that these findings will lead to the elucidation of essential properties of LSC quiescence and the design of therapeutic strategies for their clinical identification and control.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.