Abstract
Background. Relapsed/refractory (R/R) acute myeloid leukemia (AML) is a largely unmet medical condition with an overall dismal outcome owing to the lack of standardized, effective treatment approaches. The only curative option for R/R AML patients (pts) is allogeneic hematopoietic stem cell transplantation (HSCT), that can be exploited in a minority of otherwise eligible pts due to poor efficacy and high toxicity of salvage regimens used to induce leukemia remission as bridge-to-transplant. Recently, a number of targeted agents with relatively favorable toxicity profiles have been explored in clinical trials for R/R AML pts. Since its first accelerated approval by the Food and Drugs Administration in 2018, the Bcl-2 inhibitor venetoclax (VEN), in combination with hypomethylating agents (HMA) or low dose cytarabine (LDAC), has steadily become the standard therapeutic approach for newly diagnosed AML in elderly or unfit pts, while its role in R/R disease is not well defined yet.
Aim. To gain insights into the potential effectiveness of VEN-based regimens in patients with R/R AML.
Patients. We retrospectively analyzed the clinical outcomes of 67 R/R AML pts treated at our Institution with venetoclax-based regimens on an off-label basis, between March 2018 and April 2022. Fifty-eight pts (87%) carried a de-novo AML diagnosis, 6 pts (9%) had AML secondary to myelodysplastic syndrome and 3 pts (4%) had therapy-related AML. Median age was 58y (33-74y); 30 pts (45%) were aged >60, while 7 pts (10%) were aged >70y at VEN initiation. Thirty-eight pts (56%) were treated for first (n=32) or second relapse (n=6); last complete remission (CR) duration was <6 mo in 18 pts, 6-12 mo in 16 pts and >12 m (12-56) o in 4 patients; 11 pts (16%) had relapsed after HSCT; remaining 18 pts (27%) were treated for primary refractory disease. All pts received conventional immunophenotypic and cytogenetic characterization and at least NPM1, FLT3, CBFB/MYH11 and RUNX1/RUNX1T1 mutation/rearrangement detection. Next-generation sequencing (NGS) data were available for 56 pts (83%); specifically, 40 pts (primary refractory, n=18; relapsed, n=22) had NGS analysis carried out on bone marrow samples shortly before VEN initiation, while in 16 pts NGS was carried out on baseline diagnostic specimens. VEN partner drug was azacitidine in 45 pts (68%), LDAC in 15 (22%) and decitabine in 7 (10%). Thirty-nine pts (58%) were treated with an intention-to-transplant (ITT). Median follow-up duration was 11.8 mts (1.1 - 44).
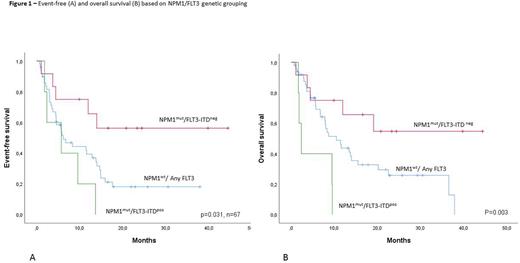
Results. Overall, we report a composite complete response (CCR) rate of 55% (n=37); 10 of complete responders (27%) had incomplete peripheral counts recovery. Minimal residual disease (MRD) negativity was achieved in 21/37 pts (56%). All CR invariably occurred during the first 8 weeks of treatment. Although it did not reach significance level (p=0.065), there was considerable variation in CCR rate among treatment settings: relapsed pts, 66%; primary refractory, 50%; post-HSCT relapse, 27%. Conventional risk stratification parameters (age, leukocyte counts, ELN 2017 risk category, NPM1/FLT3 mutational status, secondary disease, last CR duration) were poorly predictive of CR probability. A positive trend towards higher CCR rate, although not statistically significant, was found for NPM1 (CCR rate: 57%) and IDH1/2 mutation (66%) while FLT3/Ras pathway mutations were detrimental (23%). Median overall survival (OS) but not event-free survival (EFS), differed significantly among settings: relapsed pts, 11.9 mo; refractory, 8.6 mo; post-HSCT, 6.7 mo. (p=0.030). Stratifying pts based on NPM1 and FLT3-ITD status resulted in markedly different EFS: NPM1mut/FLT3-ITDneg, not reached; NPM1wt/FLT3any, 6.2 mo; NPM1mut, FLT3-ITDpos, 3.4 mo (p=0.031); as well as different OS: NPM1mut/FLT3-ITDneg, not reached; NPM1wt/FLT3any, 10.5 mo; NPM1mut, FLT3-ITDpos, 5.6 mo (p=0.003). Among the ITT population, HSCT actualization rate was 66%. Four pts are currently long-term survivors with follow-up exceeding 24 mo without receiving HSCT; notably, they all carried a NPM1mut/FLT3-ITDneg/Ras pathwayneg genotype.
Conclusions: Overall, these findings suggest a role for venetoclax-based regimens in R/R AML patients and support the design of prospective studies.
Disclosures
Scappini:Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees. Mannelli:Blueprint: Speakers Bureau; Novartis: Speakers Bureau. Guglielmelli:Novartis, Abbvie: Other: Other member of advisory board, speaker at meeting. Vannucchi:Abbvie: Membership on an entity's Board of Directors or advisory committees; AOP Orphans Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; BluePrint: Membership on an entity's Board of Directors or advisory committees, Other: NA; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees, Other: NA; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Gianfaldoni:Abbvie: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Patients with relapsed/refractory acute myeloid leukemia included in our study had received venetoclax, in association with azacitidine, decitabine or low-dose cytarabine in order to achieve a complete remission.
Author notes
Asterisk with author names denotes non-ASH members.