Abstract
Purposes Validate the predictive scoring systems for molecular responses in a large population of persons with chronic phase chronic myeloid leukemia (CML).
Methods Data from 1,297 consecutive subjects with chronic phase CML receiving initial imatinib at one centre (Beijing, China) were interrogated as the imatinib internal validation dataset and results compared with 2,815 similar subjects from 65 other centers in China as the imatinib external validation dataset. These datasets were used to evaluate performance of prediction of major molecular response (MMR) and MR4 using MMRscore and MR4score calculated as described (https://www.nature.com/articles/s41375-022-01616-y). To further evaluate the performance of the predictive scoring systems in subjects receiving the second generation tyrosine kinase inhibitor (2G-TKI), data from 284 subjects with chronic phase CML receiving a 2G-TKI therapy at one centre (Beijing, China) were collected as the 2G-TKI internal validation dataset; 807 similar subjects from 65 other centers in China, the 2G-TKI validation dataset. Propensity score matching (PSM) was used to adjust the differences in baseline co-variates between subjects initially receiving imatinib or a 2G-TKI.
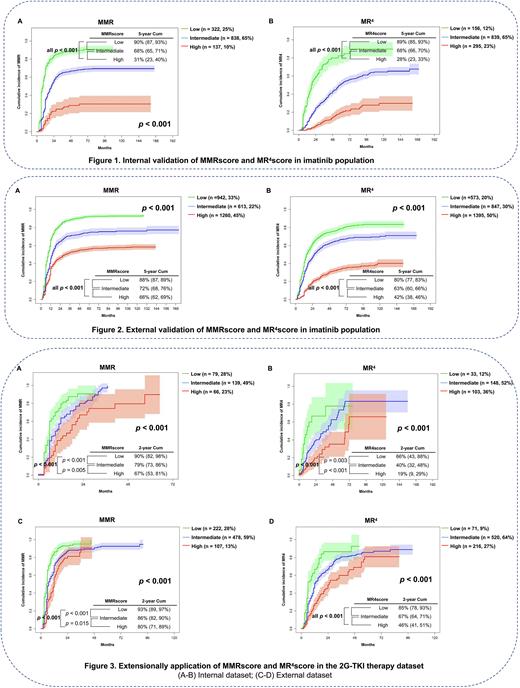
Results In the imatinib internal validation dataset, 322 (25%), 838 (65%) and 137 (10%) subjects were classified into the MMR low-, intermediate- and high-risk cohorts. 5-year cumulative incidences of MMR were 90% (95% Confidence Interval [CI] 87, 93%), 68% (65, 71%) and 31% (23, 40%; p < 0.001; Figure 1A). 156 (12%), 839 (65%) and 295 (23%) subjects were classified as MR4 low-, intermediate- and high-risk cohorts. 5-year cumulative incidences of MR4 were 89% (85, 93%), 68% (66, 70%) and 28% (23, 33%; p-value < 0.001, Figure 1B). In the imatinib external validation dataset, 942 (33%), 613 (22%) and 1260 (45%) subjects were classified as MMR low-, intermediate- and high-risk cohorts. 5-year cumulative incidences of MMR were 88% (87, 89%), 72% (68, 76%) and 66% (62, 69%) (p < 0.001, Figure 2A). 573 (20%), 847 (30%) and 1395 (50%) subjects were classified as MR4 low-, intermediate- and high-risk cohorts. 5-year cumulative incidences of MR4 were 80% (77, 83%), 63% (60, 66%) and 42% (38, 46%, p-value < 0.001; Figure 2B). In the 2G-TKI therapy internal validation dataset, 79 (28%), 139 (49%) and 66 (23%) subjects were classified as MMR low-, intermediate- and high-risk cohorts. 2-year cumulative incidences of MMR were 90% (82, 98%), 79% (73, 86%) and 67% (53, 81%; p < 0.001[ Figure 3A). 33 (12%), 148 (52%) and 103 (36%) subjects were classified as MR4 low-, intermediate- and high-risk cohorts. 2-year cumulative incidences of MR4 were 66% (43, 88%), 40% (32, 48%) and 19% (9, 29%; p < 0.001, Figure 3B). In the external 2G-TKI validation dataset, 222 (28%), 478 (59%) and 107 (13%) subjects were classified as MMR low-, intermediate- and high-risk cohorts. 2-year cumulative incidences of MMR were 93% (89, 97%), 86% (82, 90%) and 80% (71, 89%; p < 0.001, Figure 3C). 71 (9%), 520 (64%) and 216 (27%) subjects were classified as MR4 low-, intermediate- and high-risk cohorts. 2-year cumulative incidences of MR4 were 85% (78, 93%), 67% (64, 71%) and 46% (41, 51%) (p-value < 0.001, Figure 3D). By PSM analyses, subjects receiving initial 2G-TKI had the higher cumulative incidences of MMR and MR4 independent of MMR and MR4 risk cohort (p < 0.001 - 0.015).
Conclusions The MMRscore and MR4score predictive scoring systems for molecular responses accurately predict cumulative incidences of MMR and MR4 with good discrimination in persons receiving initial imatinib or a 2G-TKI. Subjects receiving initial 2G-TKI therapy had higher cumulative incidences of MR and MR4 in each risk cohorts.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.