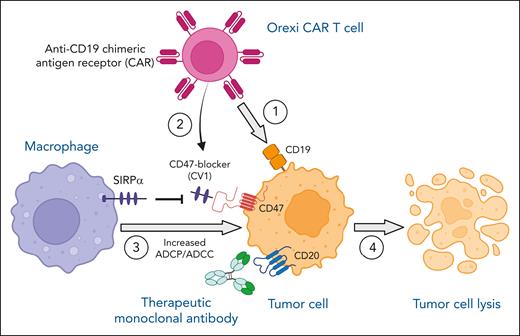
In this issue of Blood, Dacek et al present a novel strategy to enhance therapeutic antibody-dependent killing of tumors through additional infusion of “Orexi” chimeric antigen receptor (CAR) T cells engineered to locally secrete a small anti-CD47 blocking agent that disrupts antiphagocytic signaling induced by tumor cell–CD47 binding to macrophage signal-regulatory protein (SIRP) α.1 This enabled reversal of the tumor immunosuppressive microenvironment, thereby increasing macrophage-mediated antibody-dependent cellular phagocytosis (ADCP) or antibody-dependent cellular cytotoxicity (ADCC) and culminating in increased tumor cell lysis in a mouse model.
Therapeutic antibodies have revolutionized the field of cancer immunotherapy. To date, >100 monoclonal antibodies (mAbs) have been approved by the US Food and Drug Administration for the treatment of various human disorders, including cancer.2 mAbs (IgG or in other formats, such as fragments or bispecifics) specifically bind to their target antigens and elicit various effector mechanisms, such as ADCP, ADCC, and complement-dependent cytotoxicity (CDC).3
Another transformative immunotherapeutic approach has been adoptive transfer of CAR T cells targeting CD19.4 CAR T cells are T cells that have been genetically altered to express a CAR, which can target a tumor. A CAR is composed of the antigen-binding domains of an antibody fused to T-cell receptor signaling machinery. In 2010, the first case was reported of a patient with refractory lymphoma who was successfully treated with anti-CD19 CAR autologous T cells, resulting in regression of the lymphoma.5
High response rates have been reported for both mAb and CAR T-cell cancer therapy; however, treatment refractoriness and disease relapses still occur and pose an ongoing clinical challenge. This can be due to several antigen-escape mechanisms that are utilized by the tumor.6 In addition, the tumor microenvironment may be highly immunosuppressive, restricting the therapeutic efficacy of CAR T cells. The tumor microenvironment consists of immunosuppressive cytokines (eg, interleukin 10 [IL-10]) and various cells, including myeloid-derived suppressor cells, regulatory T cells, and macrophages.7 Antibody-dependent killing of tumor cells can also be limited by the engagement of tumor cell–CD47 expression and the inhibitory SIRPα on myeloid cells.8
In the current study, Dacek et al elegantly address the issues of immunotherapy refractoriness, disease relapse, and the hurdle of an immunosuppressive tumor microenvironment, by investigating the additive effects of anti-CD19 CAR T cells with an anti-CD20 mAb (rituximab), also found on the cell surface of most cancer B cells. Nonobese diabetic severe combined immunodeficiency γ (NSG) mice were engrafted with lymphoma tumor cells (CD19+/CD20+) and infused with anti-CD19 CAR T cells in combination with rituximab. This resulted in regression of the tumor and in a significantly increased survival rate, compared with CAR T cells with control mAb or rituximab alone. Subsequently, the authors investigated if the additive effects of CAR T cells and rituximab could be further strengthened through engineering of CAR T cells to secrete a CD47-SIRPα checkpoint blocker. These CAR T cells were termed “Orexi” CAR T cells, as they are designed to improve cytotoxicity via enhanced orexigenic activity through ADCP. Blocking the CD47-SIRPα signaling pathway with anti-CD47 has previously been shown to enable phagocytosis of human non-Hodgkin lymphoma cells in mice and to synergize with rituximab, reducing the lymphoma burden and improving survival.9 A CD47-SIRPα checkpoint blocker, a truncated SIRPα mimic, CV1, was used in the current study. Orexi CAR T cells did not cause any detrimental effects or impair activation, such as cytolytic activity, apoptosis, and signs of rejection. In addition, CV1 secretion did not alter the immune function and pharmacokinetics of Orexi CAR T cells. CV1 secretion by Orexi CAR T cells was shown to increase in response to CAR stimulation by antigen-positive tumor cells. Subsequently, Orexi CAR T-cell–treated mice had a reduced tumor burden compared with wild-type (WT) treated mice. On the basis of previous studies, it was postulated that this response may be potentiated by mAb therapy. Indeed, rituximab alone elicited a minor anti-tumor response, but this was greatly increased by combination treatment with Orexi CAR T cells. More important, the combination of Orexi CAR T cells and rituximab significantly increased the overall median survival of the mice (86 days) compared with mice infused with WT CAR T cells in combination with soluble CV1 and rituximab (59 days) or CV1 plus rituximab (47 days). These experiments clearly demonstrated the synergistic therapeutic effects of CAR T cells and mAb therapy, which was further enhanced by Orexi CAR T-cell secretion of CV1. These effects were suggested to be due to increased ADCP activity, as in vivo depletion of macrophages with clodronate abrogated the therapeutic effect of rituximab treatment in the tumor-engrafted NSG mice. In addition, in vitro cellular coculture experiments were performed with anti-inflammatory M2 macrophages and CAR T cells, which suggested that Orexi CAR T cells may decrease the levels of immunosuppressive IL-10 and increase the levels of interferon gamma, compared with WT CAR T cells. This suggests that Orexi CAR T cells are able to reverse the immunosuppression of M2 macrophages, thereby enhancing immune activation and tumor cell lysis. The murine in vivo experiments were performed in the intraperitoneal cavity, which is enriched in macrophages, supporting ADCP. Additional experiments, however, are required to assess the precise contribution of ADCP, ADCC, and CDC in a more typical in vivo setting, as NSG mice are deficient in mature lymphocytes and natural killer cells and are relatively deficient in complement.
In summary, Dacek et al report a potential breakthrough in the field by presenting a novel strategy to enhance the efficacy of therapeutic mAb cancer immunotherapy by addition of Orexi CAR T cells, which locally secrete the CD47-SIRPα checkpoint blocker CV1, which upends the immunosuppressive tumor microenvironment by enabling macrophage ADCP/ADCC (see figure). More importantly, the strategy of local secretion of CV1 bypasses the CD47 sink present on all cells in the body and may, thereby, prevent systemic toxicities. Further preclinical validation is warranted, followed by clinical trials investigating this exciting and promising approach, which has the potential to overcome the limitations of monotherapy, thereby preventing therapy refractoriness and disease relapse.
Anti-CD19 Orexi CAR T cells, secreting a CD47-SIRPα checkpoint inhibitor (CV1), potentiate antibody-mediated (anti-CD20; rituximab) tumor cell lysis via enhancing macrophage-mediated ADCP/ADCC. On administration of anti-CD19 Orexi CAR T cells and therapeutic anti-CD20 mAb rituximab, anti-CD19 CAR engages CD19 on the tumor cell surface (1). Local CV1 secretion by Orexi CAR T cells is enhanced because of CAR stimulation by CD19-positive tumor cells (2). CV1 binds to CD47 expressed by tumor cells and blocks the signaling pathway between inhibitory SIRPα on macrophages and tumor-CD47, enabling increased macrophage-mediated ADCP/ADCC (3). Overall, this synergistic approach of combining CAR T-cell therapy, local CD47 blockade, and mAb therapy results in increased tumor cell lysis (4). Figure created with BioRender.com.
Anti-CD19 Orexi CAR T cells, secreting a CD47-SIRPα checkpoint inhibitor (CV1), potentiate antibody-mediated (anti-CD20; rituximab) tumor cell lysis via enhancing macrophage-mediated ADCP/ADCC. On administration of anti-CD19 Orexi CAR T cells and therapeutic anti-CD20 mAb rituximab, anti-CD19 CAR engages CD19 on the tumor cell surface (1). Local CV1 secretion by Orexi CAR T cells is enhanced because of CAR stimulation by CD19-positive tumor cells (2). CV1 binds to CD47 expressed by tumor cells and blocks the signaling pathway between inhibitory SIRPα on macrophages and tumor-CD47, enabling increased macrophage-mediated ADCP/ADCC (3). Overall, this synergistic approach of combining CAR T-cell therapy, local CD47 blockade, and mAb therapy results in increased tumor cell lysis (4). Figure created with BioRender.com.
Conflict-of-interest disclosure: The author declares no competing financial interests.