Key Points
AIHA in pregnancy/puerperium was severe, required therapy (steroids ± IVIG) in nearly all cases and blood transfusions in half of patients.
Adverse maternal-fetal outcomes occurred in ∼1 of 4 cases, with fetal complications being more frequent and severe than in healthy women.
Abstract
Relapsing or occurring de novo autoimmune hemolytic anemia (AIHA) during pregnancy or puerperium is a poorly described condition. Here, we report 45 pregnancies in 33 women evaluated at 12 centers from 1997 to 2022. Among the 20 women diagnosed with AIHA before pregnancy, 10 had a relapse. An additional 13 patients developed de novo AIHA during gestation/puerperium (2 patients had AIHA relapse during a second pregnancy). Among 24 hemolytic events, anemia was uniformly severe (median Hb, 6.4 g/dL; range, 3.1-8.7) and required treatment in all cases (96% steroids ± intravenous immunoglobulin, IVIG, 58% transfusions). Response was achieved in all patients and was complete in 65% of the cases. Antithrombotic prophylaxis was administered to 8 patients (33%). After delivery, rituximab was administered to 4 patients, and cyclosporine was added to 1 patient. The rate of maternal complications, including premature rupture of membranes, placental detachment, and preeclampsia, was 15%. Early miscarriages occurred in 13% of the pregnancies. Fetal adverse events (22% of cases) included respiratory distress, fetal growth restriction, preterm birth, AIHA of the newborn, and 2 perinatal deaths. In conclusion, the occurrence of AIHA does not preclude the ability to carry out a healthy pregnancy, provided close monitoring, prompt therapy, and awareness of potential maternal and fetal complications.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2034.
Disclosures
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning Objectives
Upon completion of this activity, participants will:
Describe autoimmune hemolytic anemia (AIHA) severity and treatment during pregnancy and puerperium, according to the results of the first multicenter international cohort study evaluating AIHA, which reported on 45 pregnancies in 33 women followed at 12 centers from 1997 to 2022
Determine the impact of AIHA on maternal and fetal outcomes during pregnancy and puerperium, according to the results of the first multicenter international cohort study evaluating AIHA
Identify clinical implications of AIHA severity, treatment need, and impact on maternal and fetal outcomes during pregnancy and puerperium, according to the results of the first multicenter international cohort study evaluating AIHA
Release date April 20, 2023; Expiration date: April 20, 2024
Introduction
It has long been known that pregnancy is associated with perturbations of the immune system homeostasis, including autoimmune phenomena. It has been estimated that1 in 50 000 pregnancies may develop antierythrocyte autoantibodies with different phenotypic expressions. However, not all patients with antierythrocyte autoantibodies develop overt autoimmune hemolytic anemia (AIHA).1 The latter is a rare disease with an estimated incidence of 0.8 to 3 out of 100 000 individuals per year and is predominant in females.2 De novo or relapsing AIHA during pregnancy or puerperium is even rarer, with mainly case reports, mostly of warm immunoglobulin G (IgG) type.3 Very little is known about disease characteristics, management, maternal-fetal outcomes, and safety concerns limit treatment. Here, we report the results of the first multicenter international cohort study evaluating AIHA in the setting of pregnancy/puerperium. We aimed to delineate AIHA severity, treatment need, and the impact on maternal and fetal outcomes.
Study design
Patients were retrospectively collected from 12 tertiary hematology centers with expertise in AIHA diagnosis and management in Italy, Denmark, France, the Netherlands, the United Kingdom, the United States, and Spain. The inclusion criteria were the diagnosis of AIHA, classified according to current guidelines,4 in women of childbearing potential who had: (1) AIHA diagnosed before pregnancy; and (2) de novo AIHA, occurring during pregnancy or puerperium. The study period ranged from 1997 to 2022.
Clinical and hematological parameters were collected during AIHA flares during pregnancy or postpartum/puerperium. AIHA therapies performed before and after pregnancy were registered. Response to therapy was categorized as partial (ie, Hb ≥ 10 g/dL or increase of at least 2 g/dL from baseline) or complete (Hb ≥ 12 g/dL). Pregnancy outcomes were systematically recorded and compared with those of a control group of 56 healthy pregnant women randomly chosen among the 5720 pregnancies registered at the Milan facility during the study period and matched by the decade of pregnancy and age at pregnancy. This study was conducted following the Declaration of Helsinki and approved by the local ethics committees.
Results and discussion
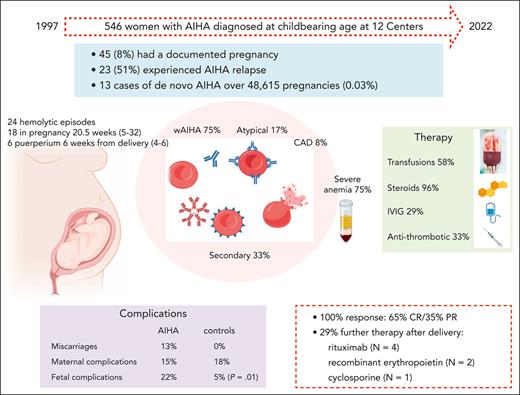
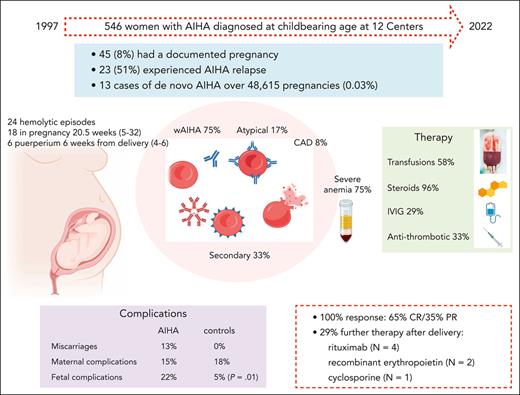
Among 546 women with AIHA diagnosed at childbearing age in the study period, 45 (8%) had a documented pregnancy and 23 (51%; 95% CI, 39-92) patients experienced AIHA relapse. In the same facilities, 48 615 pregnancies were registered, and only 13 cases of de novo AIHA were observed (0.03%; 95% CI, 0.005-0.04) (supplemental Table 1; available on the Blood website).
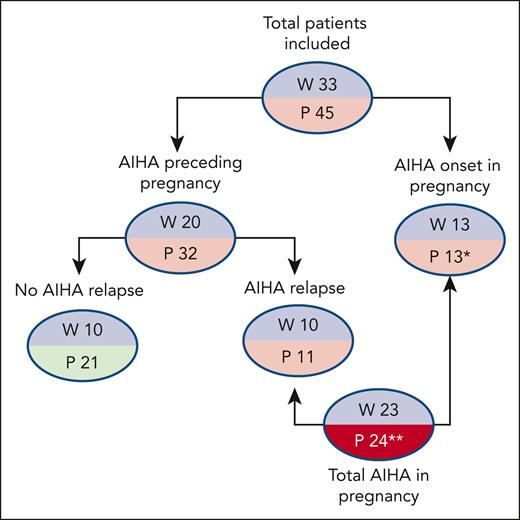
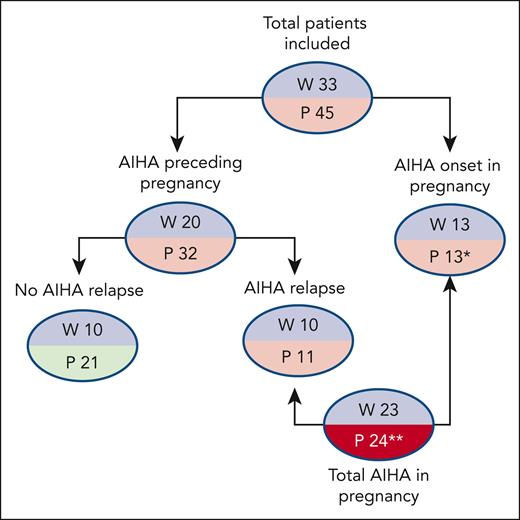
Detailed clinical and laboratory data were available for 45 pregnancies in 33 women (Table 1). Eight patients had an associated condition, including systemic lupus erythematosus with antiphospholipid syndrome (N = 1), hypothyroidism (N = 1), β-thalassemia trait (N = 2), ANA positivity >1:160 (N = 3), and type 1 diabetes mellitus (N = 1), and 2 of the 17 cases tested had positive antiphospholipid antibodies. As shown in Figure 1, AIHA diagnosis predated pregnancy in 20 women and required at least 1 therapy line in all of them and ≥2 lines in 13 (rituximab, N = 9; cytotoxic immunosuppressants, N = 6; splenectomy, N = 5). Among these 20 patients, 8 relapsed during pregnancy, 2 during puerperium, and 8 were on active treatment at the time of pregnancy (steroids, N = 6; cyclosporine, N = 1; steroids plus azathioprine, N = 1; the latter discontinued after a positive pregnancy test). One patient with a history of AIHA experienced a relapse of immune thrombocytopenia (ITP) during pregnancy.
Patient disposition. ∗Two patients had AIHA relapse during a second pregnancy; 1 patient had a second pregnancy without AIHA relapse; ∗∗2 patients had a relapse in a second pregnancy. P, number of pregnancies; W, number of women.
Patient disposition. ∗Two patients had AIHA relapse during a second pregnancy; 1 patient had a second pregnancy without AIHA relapse; ∗∗2 patients had a relapse in a second pregnancy. P, number of pregnancies; W, number of women.
An additional 9 patients developed de novo AIHA during gestation, and 4 developed AIHA in the puerperium. Two patients had AIHA onset during the puerperium of the first pregnancy and relapse during the second pregnancy (for one of these, data are available only for the second pregnancy).
We observed 24 hemolytic events in 23 women during pregnancy (median gestational week, 20.5; range 5-32) or puerperium (median week from delivery, 6; range, 4-6). Median Hb was 6.4 g/dL (range, 3.1-8.7), LDH 599 IU/L (range, 269-1631), unconjugated bilirubin 2.8 mg/dL (range, 1.8-6), reticulocytes 255 × 109/L (range, 113-780), and all subjects had haptoglobin consumption. Management consisted of blood transfusions (N = 14, 58%) and prompt establishment of steroid therapy (N = 23, 96%). Steroid treatment consisted of methylprednisolone (N = 6) or prednisone/prednisolone (N = 18) at a median dose of 1 mg/kg per day (range, 0.5-1.5 mg/kg per day); only 1 patient received 1 g/day per 3 days. All patients responded (15 CR and 8 PR) with a median time to response of 14 days (range, 7-26) and a median treatment duration of 17 weeks (range, 4-80) (supplemental Tables 2 and 3). IVIG were used in 7 of 24 AIHA flares (6 during pregnancy and 1 during puerperium), all but 1 with Hb < 8 g/dL; doses ranged from 0.4 g/kg for 5 days to 1 g/kg for 2 days, and all patients were receiving concomitant steroids making it difficult to discriminate the contribution to the overall response (supplemental Tables 2 and 3). One patient with de novo AIHA during pregnancy received transfusion support exclusively during gestation, and was treated postpartum. Antithrombotic prophylaxis was administered to 8 patients (4, heparin; 2, aspirin; and 2, both; 33%) because of hemolysis (N = 5, 2 patients previously splenectomized), antiphospholipid antibody positivity and previous miscarriages, gestosis, and COVID-19 infection (N = 1 each) (supplemental Tables 2 and 3). No prophylaxis was administered for Pneumocystis jirovecii pneumonia. In 9 of the 18 patients with AIHA relapse during pregnancy, hemolysis persisted after delivery and was managed primarily with steroids with the addition of rituximab (N = 4), recombinant erythropoietin (N = 2), and cyclosporine (N = 1), with no apparent effect on hemolysis duration.
In 7 of 45 pregnancies, maternal complications occurred (15%), including 1 premature rupture of membrane, 1 placental detachment, 2 preeclampsia, 1 severe SARS-CoV2 infection during immunosuppressive therapy, 1 postpartum infection, and 1 biliary colic. Notably, 5 of the 7 complications occurred during active hemolysis. Furthermore, 6 early miscarriages were reported: 1 in a patient with de novo AIHA during pregnancy and 5 in women with refractory AIHA who never achieved complete control of hemolysis. Ten fetal adverse events (10 out of 45, 22%) were reported: 1 transient respiratory distress of the newborn in a mother with active AIHA, 3 cases of fetal growth restriction, 2 preterm births, 1 epilepsy, 1 AIHA of the newborn (requiring IVIG, transfusions, and plasma exchange), and 2 perinatal deaths. The latter occurred in women undergoing active AIHA therapy and was secondary to massive placental detachment (1 had SARS-CoV-2 infection). Fetal complications were comparable between IgG-mediated AIHA and other AIHA forms, and no correlation with the associated condition was documented. DAT results were available for 11 newborns (46%) that were positive in 2 for immunoglobulin M and immunoglobulin A (supplemental Tables 2 and 3).
Maternal complications were comparable between the AIHA and control group (15% in AIHA vs 18% in healthy pregnancies), whereas fetal adverse events were more frequent and severe in AIHA pregnancies vs controls (22% vs 5%, P = .01).
Here, we show that de novo/relapsing AIHA during pregnancy/puerperium is a severe event that requires treatment in all patients. In particular, hemolytic relapses were observed in 50% of women with an AIHA diagnosis preceding pregnancy, suggesting close monitoring in these patients. Importantly, severe maternal and fetal complications occurred in a significant proportion of the cases and were associated with active hemolysis. The rate of maternal events registered in our AIHA series was similar to that in the control group and the general population (0.07%-37% depending on the severity and the presence of comorbidities).5,6 In contrast, fetal complications (22%) were more frequent than in the control group (5%) and in the general population, where preterm birth is the most common (8%-10%).7-9 Particularly, stillbirth was observed in 4% of cases (11%, considering pregnancies with hemolytic events), similar to a recent meta-analysis,3 whereas no fetal deaths occurred in the control group. Because IgG isotypes cross the placenta, newborns of mothers with warm AIHA may have positive direct Coombs test and hemolysis, as observed in 59% of neonates in the meta-analysis. In contrast, only 1 neonatal AIHA was observed in our series. In addition, free Hb release and nitric oxide depletion during active hemolysis may contribute to endothelial alteration, microthrombi formation, and placental dysfunction in AIHA. Antithrombotic prophylaxis was administered in only 8 patients, mainly because of active hemolysis, a previous splenectomy, and concomitant antiphospholipid syndrome in 1 woman. No overt thromboses were reported. Given the increasing awareness of AIHA-associated thrombotic risk, a risk/benefit analysis of thromboprophylaxis should be pursued in the setting of pregnancy/puerperium, particularly in the presence of additional risk factors.
Epidemiologically, autoimmunity complicates 0.9% to 10% of pregnancies,1 but the incidence of AIHA is difficult to establish. Accounting for the possible bias represented by registering only severe events that required hematology consultation, we observed 24 pregnancy-associated hemolytic episodes over 48 615 pregnancies in up to 25 years. In the meta-analysis, 51 cases were registered worldwide, most occurring in the second or third trimester (83.3%), and DAT-negative hemolysis was reported in 41% of cases.3 In our study, negative DAT occurred in 13%. The differential diagnosis of DAT-negative AIHA in pregnancy is complex and requires the exclusion of other potential causes of hemolysis (physiologic conditions such as hemodilution and nutrient deficiency; chronic preexisting ones, that is, hemoglobinopathies, congenital hemolytic anemias, and so on; and acute severe forms, such as preeclamptic syndromes and microangiopathies), and is established based on steroid response.
Regarding AIHA relapse in a second pregnancy, a rate of 40% was reported in the meta-analysis and 2 cases in our study,3 suggesting increased attention and close monitoring in women with previous pregnancy-associated AIHA.
Concerning treatment, AIHA responded well to steroids; red blood cell transfusions administered to more than 50% of our patients appeared safe and should not be delayed, especially in case of fetal distress. In addition, IVIG, used in 7 patients, may be considered for refractory or very severe cases. In fact, no data are available on novel biologic drugs in this setting, unlike other hematologic and rheumatologic diseases (eg, eculizumab in paroxysmal nocturnal hemoglobinuria,10 thrombopoietin receptor agonists in ITP,11 and anti-TNF-α in lupus12). Rituximab, which is widely used in AIHA, is known to cross the placenta and possibly induce B-cell depletion in the fetus. In our series, it was administered only after delivery. In the recent consensus for ITP and rheumatologic diseases, rituximab has been included among drugs with insufficient documentation regarding fetal safety.13,14 However, its use may be considered in severe refractory cases, weighing the risks against the benefits.15 In conclusion, although this study does not allow for specific recommendations, it provides insight into pregnancy outcomes in AIHA. Data suggest that the occurrence of AIHA does not preclude the ability to carry a healthy pregnancy, provided that close monitoring and prompt therapy are ensured, and with awareness of potential maternal and fetal complications.
Acknowledgments
The authors acknowledge the editorial assistance provided by Francesca Cappellini and Valentina Attanasio (Polistudium SRL, Milan, Italy).
The open access fee was paid by the Italian Ministry of Health, Current Research Grant.
Authorship
Contribution: B.F., M.B., and W.B. conceived the study and wrote the manuscript; and all authors provided patient follow-up, collected the data, and revised the manuscript for important intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruno Fattizzo, Hematology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, University of Milan, via Francesco Sforza 35, 20122, Milan, Italy; e-mail: bruno.fattizzo@unimi.it.
References
Author notes
Data are available from the corresponding author, Bruno Fattizzo (bruno.fattizzo@unimi.it), upon reasonable request.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.