Key Points
Endogenous plasma protein anticoagulants limit clot formation by inhibiting FIXa activity.
Delaying FIXa inhibition using zymogen-like variants increases the potency of administered FIX in hemophilia B models.
Abstract
Factor IXa (FIXa) plays a pivotal role in coagulation by contributing to FX activation via the intrinsic pathway. Although antithrombin (AT) and other plasma inhibitors are thought to regulate FIXa procoagulant function, the impact of FIXa inhibition on thrombin generation and clot formation in vivo remains unclear. Here, we generated FIXa variants with altered reactivity to plasma inhibitors that target the FIXa active site but maintain procoagulant function when bound to its cofactor, FVIIIa. We found that selected FIXa variants (eg, FIXa-V16L) have a prolonged activity half-life in the plasma due, in part, to AT resistance. Studies using hemophilia B mice have shown that delayed FIXa inhibition has a major impact on reducing the bleeding phenotype and promoting thrombus formation following administration of FIX protein. Overall, these results demonstrate that the regulation of FIXa inhibition contributes in a major way to the spatial and temporal control of coagulation at the site of vascular injury. Our findings provide novel insights into the physiological regulation of FIXa, enhance our understanding of thrombus formation in vivo via the intrinsic pathway, and suggest that altering FIXa inhibition could have therapeutic benefits.
Introduction
Hemostasis is dependent on the coordinated action of coagulation proteases and cofactors to generate thrombin, the protease responsible for blood clot formation.1 These reactions are downregulated by the inhibition of serine proteases or inactivation of cofactor proteins.2 For the intrinsic Xase complex (factor IXa [FIXa]/FVIIIa), 1 point of regulation is the inactivation of FVIII/FVIIIa by activated protein C and A2 domain dissociation.3,4 Because complex formation of FIXa with FVIIIa on an anionic membrane surface greatly enhances FIXa activity toward FX,5 inactivation of the cofactor contributes in a major way to intrinsic Xase regulation. FIXa is also regulated by plasma anticoagulant proteins. Antithrombin (AT), protein S (PS), protein Z-dependent protease inhibitor, and protease nexin 2 inactivate FIXa to varying degrees.6-11 AT is thought to be the most important inhibitor of FIXa, and its activity is greatly enhanced by heparin or heparan sulfates.12-14 However, the rate of inhibition of FIXa by AT is slower than that of FXa or thrombin (∼40- and 125-fold, respectively),15,16 which raises the question of whether this interaction contributes to hemostatic regulation in vivo.
Insights into the physiological impact of FIXa regulation by AT can be obtained from naturally occurring FIX mutations. For example, although the FIX G149R (G317R) mutation results in severe bleeding, the G149E mutation yields a moderate phenotype (chymotrypsin numbering for the FIX catalytic domain used throughout the manuscript; FIX legacy numbering is provided in brackets as needed).17 Although both mutations impact FIX function, G149E has slightly improved activation by the extrinsic Xase complex and approximately threefold less reactivity with AT bound to pentasaccharide compared with G149R. This translated into greater thrombin generation potential of G149E vs G149R in the presence of heparin. This suggests that resistance to AT-heparin may rebalance the procoagulant deficiency and contribute to less severe bleeding. Other FIX mutations, located in the heparin and AT binding exosites, show that reduced inhibition by AT prolongs FIXa procoagulant activity half-life in plasma-based experiments;18,19 however, whether this prolongation of activity has a hemostatic benefit in vivo has not been investigated.
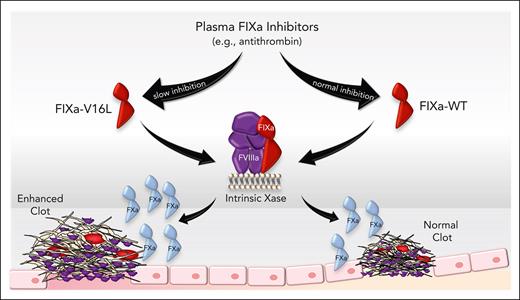
Assessing the hemostatic impact of FIXa inhibition in vivo by targeting plasma inhibitors is difficult to interpret because these anticoagulants do not exclusively act on FIXa. To address this, we used zymogen-like FIX(a) variants engineered to evade active site inhibition by plasma inhibitors, including AT. These FIX variants have an altered conformational transition from zymogen-to-protease once activated. For chymotrypsin-like serine proteases, protease formation from zymogen occurs after cleavage between amino acids 15 and 16 and insertion of the newly formed N-terminus (eg, Val16-Val17-Gly18-Gly19 for FIXa) into an activation pocket where it forms a salt bridge with Asp194.20-22 Previously, we found that mutations at residues 16 or 17 impair FXa active site function and reactivity with inhibitors such as AT.23-26 Importantly, protease activity could be rescued when FXa is bound to its cofactor, FVa. Here, we demonstrate that a similar experimental approach can be applied to FIXa to obtain molecules that adopt zymogen-like conformations. One such variant (FIX-V16L; [V181L]) has increased potency compared with wild-type (WT) FIX in hemophilia B (HB) mouse models with macro- and microcirculation injuries. This is the first in vivo experimental evidence that FIXa inhibition limits clot formation and has translational potential as a novel approach to HB treatment.
Methods
Reagents
Tissue culture reagents were obtained from Invitrogen. Spectrozyme Xa was obtained from America Diagnostica, Inc, prepared in water, and concentrations verified using E342 = 8270 M−1cm−1.27 Phospholipid vesicles composed of 75% (weight-to-weight ratio) hen egg l-α-phosphatidylcholine (PC) and 25% (weight-to-weight ratio) porcine brain l-α-phosphatidylserine (PS; Avanti Polar Lipids, Alabaster, AL) were prepared as described.28 Technothrombin thrombin calibrator and reagent RB were from Diapharma Group Inc. The fluorogenic substrate Z-Gly-Gly-Arg-AMC was obtained from Bachem Bioscience Inc. Human FIX-deficient plasma was purchased from George King Biomedical Inc. Activated partial thromboplastin time (aPTT) reagent was purchased from Trinity Biotech. Rat anti-mouse CD41 antibody, prepared as an F(ab)2 fragment, was obtained from BD Biosciences. Mouse anti-human fibrin monoclonal antibody (clone 59D8), which cross-reacts with mouse fibrin, has been previously described.29 These antibodies were conjugated with Alexa555 or Alexa647 using the Alexa Fluor Protein Labeling Kit according to the manufacturer's instructions (Molecular Probes/Invitrogen). Affinity-purified anti-human FIX polyclonal immunoglobulin G (IgG) was purchased from Affinity Biologicals, peroxidase-conjugated affinity-purified sheep anti-human AT polyclonal IgG was obtained from Enzyme Research Laboratories, and biotinylated Glu-Gly-Arg-chloromethylketone (B-EGRCK) was from Hematologic Technologies.
Proteins
Molecular weights and extinction coefficients (E0.1% 280 nm) of various proteins used were taken as previously reported.15 All functional assays were performed at 25°C in 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 0.15 M sodium chloride, 0.1% (weight-to-volume ratio) polyethylene glycol (PEG)–8000, 5 mM calcium chloride (CaCl2), pH 7.4 (assay buffer). Detailed methods regarding preparation and activation of FIX variants as well as plasma-based and ROTEM assays are found in the supplemental Methods, which are available on the Blood website.
FX activation by FIXa variants
The rate of FXa generation was determined as previously described.30,31 For FIXa studies, plasma-derived FX (pdFX) (300 nM) was incubated with FIXa (20 nM) and PCPS (20 μM) vesicles in assay buffer for 30 minutes. For intrinsic Xase studies, FIXa (0.2 nM) was incubated with FVIIIa (40 nM) on PCPS vesicles (20 μM), and the reaction was initiated by the addition of varying concentrations of pdFX (0-800 nM) to the assay buffer for different periods of time. The reactions were quenched with 20 mM HEPES, 0.15 M sodium chloride, 0.1% (weight-to-volume ratio) PEG-8000, 50 mM EDTA, and pH 7.4. The rate of FXa generation was measured using Spectrozyme Xa (250 μM). The concentration of FXa generated was determined from a standard curve prepared using known concentrations of FXa under the same conditions. The data were analyzed using nonlinear least squares regression. Initial velocity measurements were analyzed by fitting the data (R2 ≥ 0.97) to the Henri-Michaelis-Menten equation to yield fitted values for Km and Vmax. The dependence of FVIIIa on FX activation was studied using increasing concentrations of FVIIIa (0-5 nM), FIXa (0.2 nM), and PCPS vesicles (20 μM) in assay buffer, and the reaction was initiated with pdFX (200 nM). The FXa generation rate was determined as above. The data were fitted to a quadratic binding equation.
ELISA for FIXa-AT complex formation
The rate of FIXa inhibition by AT was determined as previously described, with some modifications.32 FIXa-WT and FIXa variants (25 nM) were added to human HB plasma containing 1.33 mM GPRP, 1 μM dabigatran, and 5 mM CaCl2 at room temperature. At different time points, the reactions were stopped with 100 μM B-EGRCK. The FIXa-AT standards were prepared by incubating 500 nM FIXa, 5 μM AT, and assay buffer for 1 hour. The standard was then diluted in HB plasma to concentrations ranging from 0 to 30 nM. The FIXa-AT levels in the samples were measured using a sandwich enzyme-linked immunosorbent assay (ELISA), with purified goat–anti-human FIX polyclonal as the capture antibody and peroxidase-conjugated affinity sheep anti-human AT polyclonal IgG as the detection antibody. The reaction rate was determined by fitting the data (R2 ≥ 0.97) to a single exponential rise expression with offset.
Animal models
HB Balb/c mice have been previously described and backcrossed for >10 generations.33,34 WT Balb/c mice purchased from Jackson Laboratory were used as controls. For all experiments, the mice were 8 to 12 weeks old and weighed 25 to 30 g. Approval was obtained from the Children's Hospital of Philadelphia Institutional Animal Care and Use Committee.
In vivo vascular injury models
Tail clip and tail vein transection (TVT) assays were performed as previously described.25,35 In the tail clip assay, FIX, FIXa, or phosphate-buffered saline (PBS) was injected into the tail vein 5 minutes before injury. The tail was transected at a diameter of 3 mm and blood was collected for 10 minutes. In the TVT model, PBS, FIX, or FIXa was infused 10 minutes or 30 seconds before injury via the jugular vein. The lateral vein was transected where the tail was 2.5 mm in diameter, and blood was collected for the first 3 minutes post injury (time point, 3 minutes). The collection tube was replaced with a new prewarmed tube filled with saline every 10 minutes. If bleeding stopped, the initial wound was gently wiped every 10 minutes, and blood was collected between reinjuries (time points, 20, 30, 40, 50, and 60 minutes). In both models, quantitative assessment of blood loss was determined by measuring total hemoglobin by absorbance at 575 nm following red cell lysis and was converted to total blood loss (μL) using a standard curve. The ferric chloride (FeCl3) carotid artery injury model and laser injury model of the arterioles of the cremaster muscle were performed as described.25,26,36
Statistical analysis
Curve fitting and statistical analysis were performed using GraphPad Prism 9 software. An analysis of variance followed by a Tukey test was used to compare the experimental groups in the ROTEM, tail bleeding, and FeCl3 experiments. P < .05 was considered statistically significant.
Results
Procoagulant activity of zymogen-like FIXa variants
To alter the reactivity of FIXa with plasma inhibitors such as AT, we created FIX variants with mutations at positions 16 or 17 (181 or 182). Proteins were stably transfected into HEK293 cells, antigen levels were determined by FIX-specific ELISA, and procoagulant activity was determined by 1-stage aPTT clotting assay. We found that the variants had a range of specific activities depending on the amino acid substitution at positions 16 or 17 (supplemental Table 1). Selected variants with moderate (FIX-V17I, 67%), intermediate (FIX-V16L, 4.8%), and low (FIX-V16T, <1%) specific activities relative to FIX-WT were purified to homogeneity for further characterization. The variants were activated by FXIa at rates comparable to those of FIX-WT (supplemental Table 2). Similar results were obtained using TF/FVIIa, except for FIX-V16L, which differed from those of FIX-WT.
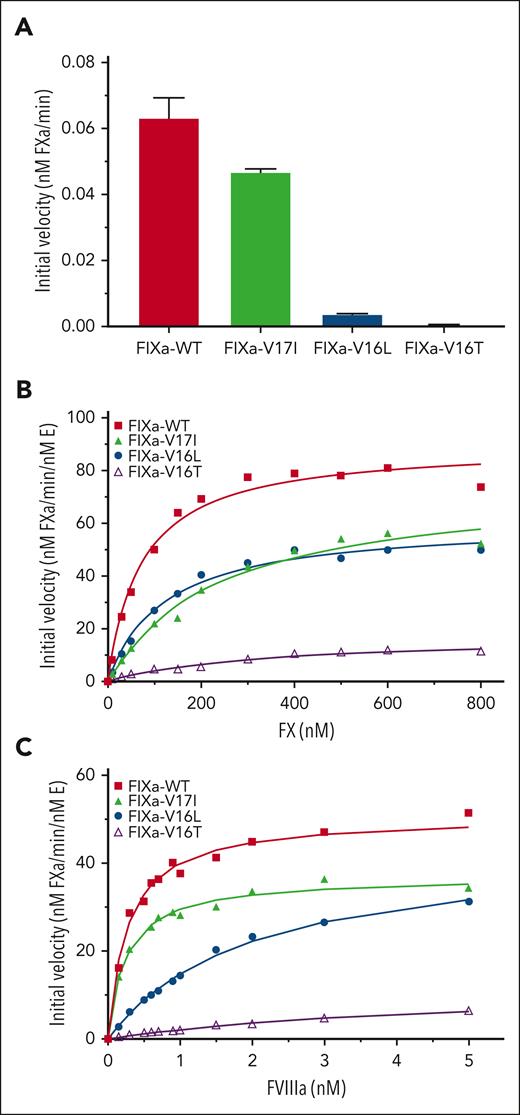
Kinetic studies in the absence of FVIIIa revealed that FIXa-V16L and FIXa-V16T had lower activity toward FX than FIXa-WT (Figure 1A), suggesting that their substrate binding pockets were in a zymogen-like conformation. FIXa-V17I had activity similar to that of FIXa-WT, suggesting a more protease-like active site conformation. These differences in activity were generally consistent when using the FIXa peptidyl substrate (supplemental Figure 1; supplemental Table 3). When these FIXa variants were assembled into the intrinsic Xase complex by addition of FVIIIa, the initial rates of FX activation by FIXa-V17I and FIXa-V16L were significantly lower than those by FIXa-WT, whereas FIXa-V16T was substantially lower (Figure 1B; Table 1). In agreement with previous studies using FXa variants,23-26 the apparent Kd of FIXa-V16L and FIXa-V16T for FVIIIa membranes was altered, whereas that of FIXa-V17I was comparable to that of FIXa-WT (Figure 1C; Table 1). These data show that modifying the FIXa zymogen-to-protease transition alters the cofactor binding to varying degrees; however, once the FIXa variant is bound to FVIIIa, catalytic activity is mostly restored.
FX activation by FIXa in the presence or absence of FVIIIa. FX activation by FIXa-WT or FIXa variants was assessed as described in “Methods.” (A) FX activation (300 nM) by 20 nM FIXa and 20 μM PCPS without FVIIIa. Data are presented as mean ± standard deviation (SD) (n = 3). (B) Enzyme kinetics of FX activation by 0.2 nM FIXa, 40 nM FVIIIa, and 20 μM PCPS. Solid lines represent the best fit of the data to the Michaelis-Menten equation (R2 ≥ 0.97). (C) FVIIIa binding to 0.2 nM FIXa was kinetically assessed using varying concentrations of FVIIIa, PCPS (20 μM), and FX (200 nM). The solid lines are the fittings of a quadratic binding equation (R2 ≥ 0.97). Data are representative of 3 independent experiments. The fitted parameters are listed in Table 2. E, enzyme.
FX activation by FIXa in the presence or absence of FVIIIa. FX activation by FIXa-WT or FIXa variants was assessed as described in “Methods.” (A) FX activation (300 nM) by 20 nM FIXa and 20 μM PCPS without FVIIIa. Data are presented as mean ± standard deviation (SD) (n = 3). (B) Enzyme kinetics of FX activation by 0.2 nM FIXa, 40 nM FVIIIa, and 20 μM PCPS. Solid lines represent the best fit of the data to the Michaelis-Menten equation (R2 ≥ 0.97). (C) FVIIIa binding to 0.2 nM FIXa was kinetically assessed using varying concentrations of FVIIIa, PCPS (20 μM), and FX (200 nM). The solid lines are the fittings of a quadratic binding equation (R2 ≥ 0.97). Data are representative of 3 independent experiments. The fitted parameters are listed in Table 2. E, enzyme.
FIXa variants are resistant to inhibition by AT
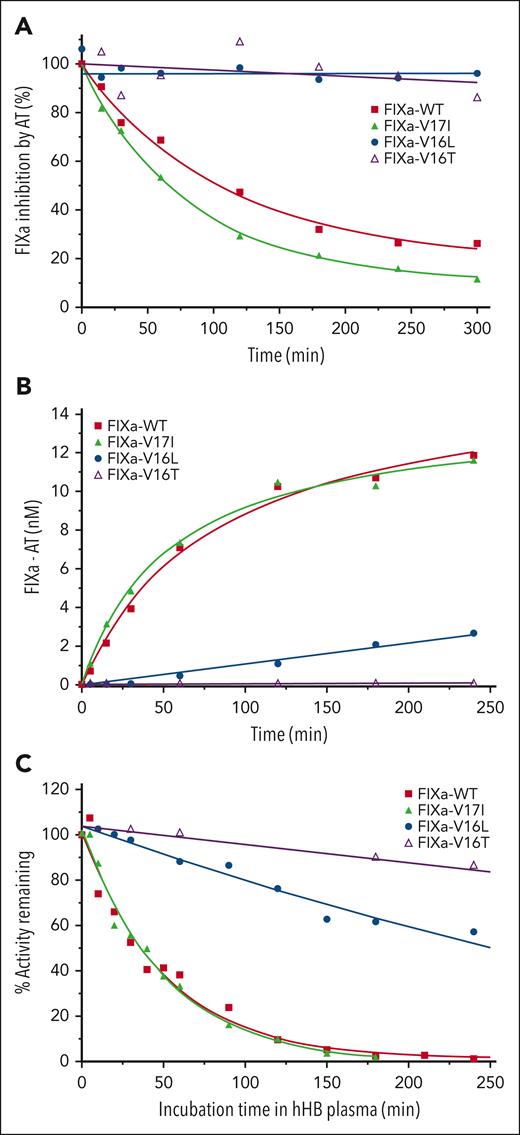
Consistent with the protease-like active site conformation, the rate of AT inhibition of FIXa-V17I was similar to that of FIXa-WT, whereas the rate of inhibition of FIXa-V16L or FIXa-V16T could not be determined (Figure 2A; Table 2). Inhibition of FIXa-WT by AT was accelerated by unfractionated heparin and fondaparinux, whereas FIXa-V16L was poorly inhibited (data not shown). We also measured the rate of FIXa-AT complex formation in plasma and obtained results comparable to those obtained using the purified system (Figure 2B; Table 2). Much less FIXa-V16L and FIXa-V16T were complexed with AT over time, indicating that these variants did not engage and were not inhibited well by AT. The rate of AT inhibition by FIXa-V16L was reduced ∼12-fold relative to FIXa-WT, whereas FIXa-V16T failed to detectably bind AT. The altered FIXa-AT reactivity was associated with prolonged procoagulant activity when FIXa-V16L and FIXa-V16T variants were added to human HB plasma. FIXa-V16L and FIXa-V16T showed substantial clotting activity compared with FIXa-WT and FIXa-V17I after 180 minutes (Figure 2C). The rate of inhibition of FIXa-V16L in plasma was reduced by ∼12-fold and could not be determined for FIXa-V16T (Table 2). These results suggested that FIXa variants with zymogen-like characteristics are protected from inhibition in the plasma environment.
Inhibition of FIXa by AT. (A) Inhibition of FIXa-WT and its variants by AT in the purified system. FIXa (500 nM) was incubated with AT (2 μM) for different periods of time, and the residual amidolytic activity was measured by the addition of SpecIXa. The residual activity is shown as a percentage of the activity corresponding to time 0, which was fitted to an exponential decay function. (B) Inhibition kinetics of FIXa-WT and its variants by AT were measured using an ELISA assay that detects FIXa-AT formation after the addition of FIXa-WT or FIXa variants (25 nM) to human HB (hHB) plasma. The solid lines represent the fit (R2 ≥ 0.97) of the points for a single exponential increase. (C) Residual activity of FIXa was determined after 1 nM FIXa-WT, -V17I, -V16L, or 30 nM -V16T were incubated in human HB plasma for different periods of time. The residual activity is shown as a percentage of the activity corresponding to time 0, which was fitted to an exponential decay function. Each curve is representative of 3 independent experiments. The fitted parameters are listed in Table 2.
Inhibition of FIXa by AT. (A) Inhibition of FIXa-WT and its variants by AT in the purified system. FIXa (500 nM) was incubated with AT (2 μM) for different periods of time, and the residual amidolytic activity was measured by the addition of SpecIXa. The residual activity is shown as a percentage of the activity corresponding to time 0, which was fitted to an exponential decay function. (B) Inhibition kinetics of FIXa-WT and its variants by AT were measured using an ELISA assay that detects FIXa-AT formation after the addition of FIXa-WT or FIXa variants (25 nM) to human HB (hHB) plasma. The solid lines represent the fit (R2 ≥ 0.97) of the points for a single exponential increase. (C) Residual activity of FIXa was determined after 1 nM FIXa-WT, -V17I, -V16L, or 30 nM -V16T were incubated in human HB plasma for different periods of time. The residual activity is shown as a percentage of the activity corresponding to time 0, which was fitted to an exponential decay function. Each curve is representative of 3 independent experiments. The fitted parameters are listed in Table 2.
FIX variants exhibit differential activity in plasma
The effect of FIX variants on human HB plasma was further studied using a thrombin generation assay (TGA). FIX-WT and FIX-V17I enhanced thrombin generation in HB plasma in a comparable manner, increasing both peak thrombin (Figure 3A) and endogenous thrombin potential (supplemental Figure 2A). In contrast, these parameters were reduced by approximately fourfold for FIX-V16L (Figure 3A; supplemental Figure 2A). This is unlikely to be because of slower activation of FIX-V16L by TF/FVIIa, because FXIa-triggered TGA (data not shown) or FIXa-triggered TGA (supplemental Figure 3) yielded similar results. To test whether suboptimal FVIIIa was generated during the assays to rescue FIXa-V16L, we repeated the experiment by adding increasing amounts of FVIII to HB plasma. Peak thrombin (Figure 3B) and endogenous thrombin potential levels (supplemental Figure 2B) for FIX-V16L substantially improved in HB plasma with increasing concentrations of FVIII, whereas the activity of FIX-WT changed modestly. This is consistent with our biochemical data showing that higher FVIIIa concentrations are needed to fully rescue the activity of FIXa-V16L because of the reduced cofactor affinity (Figure 1C). FIX-V16T had negligible procoagulant activity in TGA and no additional activity was observed with exogenous FVIIIa (data not shown), indicating that this variant could not be rescued by the cofactor in this experimental system.
Analysis of FIX-WT and FIX variants activities in human HB plasma. (A) Peak thrombin generation as a function of added FIX protein (3.9-250 nM) along with 2 pM TF/4 μM phospholipid into human HB plasma. (B) Peak thrombin generation in the presence of varying concentrations of FVIII (0-8 nM) and FIX-WT or FIX-V16L (125 nM) in the human HB plasma. Peak thrombin is shown on a semilogarithmic plot. Data are representative of 3 independent experiments.
Analysis of FIX-WT and FIX variants activities in human HB plasma. (A) Peak thrombin generation as a function of added FIX protein (3.9-250 nM) along with 2 pM TF/4 μM phospholipid into human HB plasma. (B) Peak thrombin generation in the presence of varying concentrations of FVIII (0-8 nM) and FIX-WT or FIX-V16L (125 nM) in the human HB plasma. Peak thrombin is shown on a semilogarithmic plot. Data are representative of 3 independent experiments.
Assessment of FIX variants in vivo
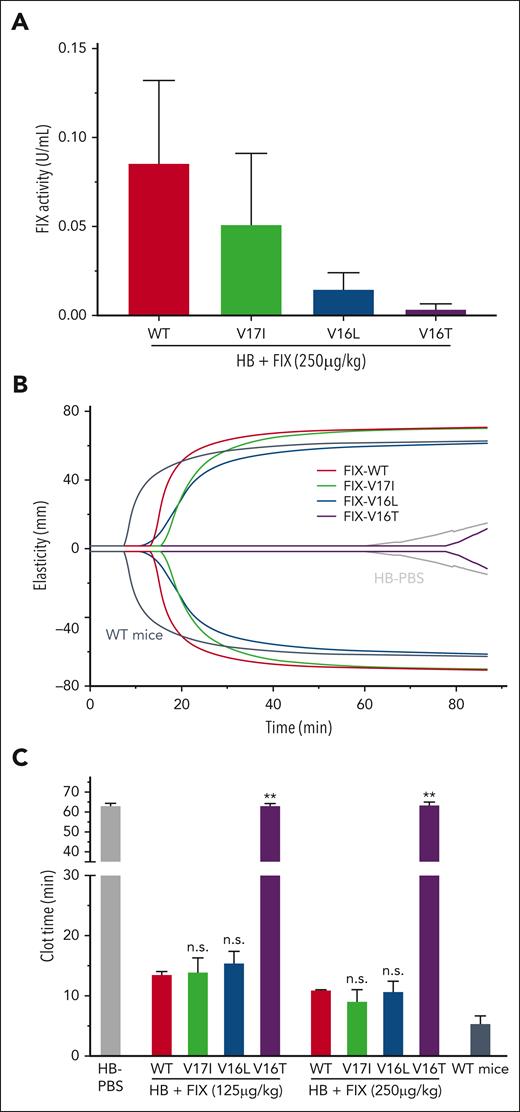
To determine whether the biochemical attributes of the FIX variants had an impact, we determined their procoagulant potency in an HB mouse model. First, we assessed the clotting activity of the variants following their injection into HB mice. As expected, the procoagulant activity of the FIX mutants in HB mice varied (Figure 4A) and mirrored that observed in human plasma (Figure 1A; supplemental Table 1). ROTEM experiments using whole blood from HB mice injected with FIX protein (250 and 125 μg/kg) show that the FIX-V16L improved clot strength parameters (Figure 4B) and significantly shortened the clot time like FIX-WT and FIX-V17I (Figure 4C). In this system, FIX-V16T had little to no effect, similar to the PBS control. To ensure that the observed differences were not owing to in vivo recovery or clearance, we measured plasma levels of FIX-V16L and FIX-WT over time after injection in HB mice. We found that the recovery of each protein (∼20%, 2 minutes after injection) and overall rate of disappearance were similar (supplemental Figure 4). Furthermore, consistent with the results from the human system, FIXa-V16L exhibited a marked prolongation of activity when introduced into mouse HB plasma (supplemental Figure 5), highlighting its resistance to protease inhibitors. Based on these data, together with those ones from purified component and plasma-based systems, we selected FIX-V16L as the most promising candidate to investigate the physiological contribution of FIXa inhibition to hemostasis in vivo.
Analysis of FIX-WT and FIX variant activities in HB mice 10 minutes after FIX injection. (A) FIX activity levels based on 1-stage aPTT FIX-specific clotting in plasma from HB mice injected with FIX proteins (250 μg/kg). Data are presented as the mean ± SD from n = 3 to 4 per group. (B) Representative ROTEM traces in whole blood obtained from HB mice that received FIX protein (125 μg/kg). Controls: WT mice (dark gray) and HB mice (gray). (C) ROTEM clot times after the administration of FIX proteins at the indicated doses in HB mice. Data are presented as mean ± SD from n = 2 to 5 per group. Adjusted P values were obtained using an analysis of variance (ANOVA) test followed by a Tukey test. ∗∗P < .001 or not significant (n.s.) represent FIX variants vs FIX-WT infused animals.
Analysis of FIX-WT and FIX variant activities in HB mice 10 minutes after FIX injection. (A) FIX activity levels based on 1-stage aPTT FIX-specific clotting in plasma from HB mice injected with FIX proteins (250 μg/kg). Data are presented as the mean ± SD from n = 3 to 4 per group. (B) Representative ROTEM traces in whole blood obtained from HB mice that received FIX protein (125 μg/kg). Controls: WT mice (dark gray) and HB mice (gray). (C) ROTEM clot times after the administration of FIX proteins at the indicated doses in HB mice. Data are presented as mean ± SD from n = 2 to 5 per group. Adjusted P values were obtained using an analysis of variance (ANOVA) test followed by a Tukey test. ∗∗P < .001 or not significant (n.s.) represent FIX variants vs FIX-WT infused animals.
In the tail clip assay, the total blood loss in PBS-injected HB mice was higher than that in WT mice (P < .001) (Figure 5A-B). Although FIX-WT and FIX-V16L (50, 75, 125, and 250 μg/kg) reduced the total blood loss, FIX-V16L was more potent than FIX-WT (50 and 75 μg/kg V16L vs WT; P = .015 and P = .005, respectively) (Figure 5A-B). The 50% effective concentration (EC50) and EC80 of FIX-V16L were 3.9- and 2.33-fold, lower than those of FIX-WT, respectively (supplemental Table 4). This increased hemostatic potency, albeit modest, in vivo is especially notable, because the specific activity of FIX-V16L was markedly lower (10 U/mg) than that of FIX-WT (207 U/mg). This result suggests that once activated in vivo, FIXa-V16L is inactivated by inhibitors (such as AT) much more slowly than FIXa-WT, similar to our in vitro observations. The enhanced hemostatic potency of FIXa-V16L compared with that of FIXa-WT is consistent with the slower rate of FIXa inactivation, resulting in a higher FIXa concentration at the site of injury, enabling more FX activation.
Hemostatic response after mechanical injury to the large vessels. (A-F) HB and WT mice infused with PBS served as the controls. Tail clip (A) or TVT assay (C) were performed in HB mice injected with FIX-WT or FIX-V16L at the indicated doses, and blood loss (μL) was collected for 10 minutes or 60 minutes, respectively. Dose-dependent bleeding reduction with FIX-V16L or FIX-WT was determined by fitting tail clip (B) and TVT (D) data to a logistic function (solid lines). The dotted line indicates the median value for the WT mice. Data are presented as the mean ± SD from n = 7 per group. Adjusted P values were obtained using an ANOVA test followed by a Tukey test. ∗P < .05, ∗∗P < .004, or n.s. represent FIX-V16L vs FIX-WT infused animals. Bleeding profile after TVT in HB mice injected with 75 μg/kg (E) or 50 μg/kg (F) FIX protein as a function of time. Each individual point represents the average blood loss (μL) between clot disruptions from 7 mice.
Hemostatic response after mechanical injury to the large vessels. (A-F) HB and WT mice infused with PBS served as the controls. Tail clip (A) or TVT assay (C) were performed in HB mice injected with FIX-WT or FIX-V16L at the indicated doses, and blood loss (μL) was collected for 10 minutes or 60 minutes, respectively. Dose-dependent bleeding reduction with FIX-V16L or FIX-WT was determined by fitting tail clip (B) and TVT (D) data to a logistic function (solid lines). The dotted line indicates the median value for the WT mice. Data are presented as the mean ± SD from n = 7 per group. Adjusted P values were obtained using an ANOVA test followed by a Tukey test. ∗P < .05, ∗∗P < .004, or n.s. represent FIX-V16L vs FIX-WT infused animals. Bleeding profile after TVT in HB mice injected with 75 μg/kg (E) or 50 μg/kg (F) FIX protein as a function of time. Each individual point represents the average blood loss (μL) between clot disruptions from 7 mice.
To assess the duration of hemostasis, a TVT model was used, which enabled blood collection for an extended period (60 minutes). In these experiments, FIX-V16L (50, 75, or 125 μg/kg) (Figure 5C-D) was more potent in reducing blood loss than FIX-WT, and reached statistical significance at lower doses (50 μg/kg, P = .0003; 75 μg/kg, P = .03) (Figure 5C), which agrees with the data from the tail clip assay. The EC50 and EC80 of FIX-V16L were 1.55- and 1.67-fold lower than that of FIX-WT, respectively (supplemental Table 4). To better understand how these proteins performed over time, blood loss was recorded between clot disruptions and grouped by time points (Figure 5E-F; supplemental Figure 6A). We observed that at each time point collected, the average total blood loss for FIX-V16L was reduced compared with that for FIX-WT (75 μg/kg, Figure 5E; and 125 μg/kg, supplemental Figure 6B). At the 75 μg/kg dose, FIX-V16L reduced blood loss to levels observed in WT mice at all time points, except at 60 minutes (Figure 5E). The differences between FIX-WT and FIX-V16L were most prominent at 50 μg/kg, where a clear reduction in blood loss was observed for ∼40 minutes after injection of FIX-V16L, whereas the effect of FIX-WT was negligible (V16L, P < .001, vs WT, P = .004) (Figure 5F). Similar results were observed when FIX proteins were injected into HB mice 1 hour before the injury (supplemental Figure 7). These data suggest that once activated, FIXa-V16L persists and contributes to the hemostatic response for longer periods than FIXa-WT.
In addition to the tail clip and TVT models, the FeCl3-induced injury model of the carotid artery was used. Compared with WT mice, HB mice had no vessel occlusion throughout the duration of the experiment (P < .001 vs WT mice) (supplemental Table 5). We found that injection of FIX-V16L into HB mice led to faster vessel occlusion compared with FIX-WT (250 μg/kg, P < .05; 125 μg/kg, P < 001, V16L vs WT). These results suggest that altering FIXa inhibition by plasma inhibitors accelerates thrombotic occlusion following vascular injury.
To further determine the contribution of FIXa inhibition to thrombus formation, we used a cremaster muscle laser-induced injury model of arterioles, which enables the real-time visualization of platelets and fibrin. In these experiments, a single injection of FIX-WT or FIX-V16L (25 μg/kg) or PBS was administered to HB mice and repeated vessel injury was induced over 60 minutes. As expected, thrombus formation was impaired in PBS-treated HB mice compared with that in WT mice at all time points (Figure 6). Initially, platelet and fibrin accumulation were restored in HB mice injected with either FIX-V16L or FIX-WT. However, over the experimental time period, differences were apparent between FIX-WT and FIX-V16L, especially as it relates to fibrin accumulation; thrombi progressively decreased in size in HB mice injected with FIX-WT, but not with FIX-V16L (Figure 6A-C). There was a modest enhancement in platelet accumulation over the course of the experiment with FIX-V16L compared with FIX-WT and WT mice (Figure 6B; supplemental Videos 1-2). A similar pattern of enhanced fibrin accumulation with FIX-V16L was observed, with peaks at 20 and 60 minutes (Figure 6C; supplemental Videos 1-2). We speculate that these enhancements in platelet and fibrin accumulation are because of the slower inhibition of activated FIX-V16L produced at the site of injury, which is particularly surprising, considering that FIX-V16L has <10% of the specific activity of FIX-WT.
Hemostatic response after laser-induced injury to arterioles. (A) Images depict representative thrombi induced at 1, 10, 20, 30, 40, and 60 minutes after injection of 25 μg/kg FIX-WT or FIX-V16L into HB mice. Platelets (red) were detected using an Alexa555-labeled rat anti-CD41 F(ab)2 and fibrin (green) Alexa647-labeled anti-fibrin antibodies; areas of overlap are yellow. (B-C) Graphs showing the median area under the curve (AUC) for CD41 or fibrin vs time curve in HB mice injected with FIX-WT or FIX-V16L (25 μg/kg), or PBS. Each time point represents the median ± SD of 4 to 5 thrombi from 4 to 5 mice.
Hemostatic response after laser-induced injury to arterioles. (A) Images depict representative thrombi induced at 1, 10, 20, 30, 40, and 60 minutes after injection of 25 μg/kg FIX-WT or FIX-V16L into HB mice. Platelets (red) were detected using an Alexa555-labeled rat anti-CD41 F(ab)2 and fibrin (green) Alexa647-labeled anti-fibrin antibodies; areas of overlap are yellow. (B-C) Graphs showing the median area under the curve (AUC) for CD41 or fibrin vs time curve in HB mice injected with FIX-WT or FIX-V16L (25 μg/kg), or PBS. Each time point represents the median ± SD of 4 to 5 thrombi from 4 to 5 mice.
To confirm that delayed FIXa inhibition was largely responsible for the enhanced procoagulant response, additional experiments were performed using injected FIXa. As observed with FIX-V16L, HB mice injected with FIXa-V16L exhibited a greater reduction in total blood loss than FIXa-WT either in a tail clip (50 μg/kg) (Figure 7A) or TVT assay (50 μg/kg V16L vs WT P = .004) (Figure 7B; supplemental Figure 8). In intravital studies, there were increased platelets and fibrin with FIXa-V16L vs FIXa-WT (10 μg/kg) (Figure 7C-D). Overall, the results from these in vivo studies investigating macro- and microcirculation suggest that FIXa inhibition by AT and other plasma protease inhibitors is an important contributor to thrombus formation, and the release of this regulatory mechanism results in an enhanced procoagulant response to vascular injury.
In vivo hemostatic function of FIXa-WT and FIXa-V16L variants. (A) Tail clip assay was performed on HB mice injected with FIXa-WT or FIXa-V16L (50 μg/kg, n = 3-7) 5 minutes before injury, and blood loss (μL) was collected for 10 minutes. (B) The TVT assay was performed on HB mice injected with FIXa-WT or FIX-V16L (50 μg/kg, n = 7) 30 seconds before injury, and blood loss (μL) was collected for 60 minutes. Data are presented as mean ± SD. Adjusted P values were obtained using an ANOVA test followed by a Tukey test. ∗∗P < .0001 or n.s. represent FIXa-V16L vs FIXa-WT infused animals. (C-D) Graphs showing the median for AUC for CD41 or for fibrin vs time curve in HB mice injected with FIXa-WT or FIXa-V16L (10 μg/kg), or PBS. Each time point represents the median ± SD of 2 to 3 thrombi from 2 to 3 mice. (panels A-D) HB or WT mice infused with PBS served as controls.
In vivo hemostatic function of FIXa-WT and FIXa-V16L variants. (A) Tail clip assay was performed on HB mice injected with FIXa-WT or FIXa-V16L (50 μg/kg, n = 3-7) 5 minutes before injury, and blood loss (μL) was collected for 10 minutes. (B) The TVT assay was performed on HB mice injected with FIXa-WT or FIX-V16L (50 μg/kg, n = 7) 30 seconds before injury, and blood loss (μL) was collected for 60 minutes. Data are presented as mean ± SD. Adjusted P values were obtained using an ANOVA test followed by a Tukey test. ∗∗P < .0001 or n.s. represent FIXa-V16L vs FIXa-WT infused animals. (C-D) Graphs showing the median for AUC for CD41 or for fibrin vs time curve in HB mice injected with FIXa-WT or FIXa-V16L (10 μg/kg), or PBS. Each time point represents the median ± SD of 2 to 3 thrombi from 2 to 3 mice. (panels A-D) HB or WT mice infused with PBS served as controls.
Discussion
The results of this study provide new insights into the regulation of the intrinsic Xase complex in vivo at the FIXa level. We showed that inhibition of FIXa has a major impact on the durability of thrombus formation. To do this, we used variants of FIXa with delayed inhibition of plasma protease inhibitors such as AT. Clot formation in several injury models was remarkably different using these variants compared with FIX-WT. These results deepen our understanding of coagulation regulation and provide new opportunities for bioengineering FIX/FIXa for therapeutic benefits. This work also shows that it is possible to make a procoagulant FIX(a) protein by delaying its inactivation. Paradoxically, this can be achieved even at the expense of a reduced specific activity.
Many plasma inhibitors target the active sites of serine proteases, including FIXa.8 We took advantage of this mechanism and selectively altered the zymogen-to-protease transition to abrogate FIXa inhibition. This experimental approach has the advantage of disrupting the dominant anticoagulant pathways that target the FIXa active site while being specific for FIXa. Direct blocking of AT, for example, affects several other coagulation factors. We created zymogen-like FIXa variants with a range of activities and reactivities with inhibitors, as in our previous studies with FX.24-26 Some variants (eg, FIX-V17I) were in a more protease-like state, with procoagulant activity and reactivity to AT, such as FIX-WT, whereas others (eg, FIX-V16T) were zymogen-like, with little procoagulant activity and reactivity to AT. FIX-V16L had a reduced active site function and was resistant to AT, but its procoagulant function (eg, activation of FX) could be almost fully rescued by binding FVIIIa and assembling it in the intrinsic Xase complex. These features make FIX-V16L a valuable tool for addressing the potential physiological role of FIXa inhibition by plasma inhibitors in vivo.
We hypothesized that if FIXa inhibition had a significant physiological function in vivo, interference with this regulatory step would enhance the procoagulant response to vessel injury. In the TVT and tail clip models, FIX-V16L was superior in reducing bleeding in HB mice than FIX-WT. This enhanced hemostatic potency is remarkable, considering that the specific activity of FIX-V16L was <10% of that of FIX-WT. Based on our biochemical characterization, we surmise that in vivo, FIXa-V16L is resistant to inhibition and accumulates in higher amounts, thereby generating more FXa and thrombin at the site of injury than FIX-WT. This model is supported by our observation that the administration of FIXa-V16L protein to HB mice resulted in a greater reduction in total blood loss compared with FIXa-WT.
Similarly, FIX-V16L demonstrated increased potency against FIX-WT in 2 in vivo clot formation models. In the FeCl3 injury model, FIX-V16L accelerated the time to occlusion compared with FIX-WT, suggesting that inhibition of FIXa activity by AT plays an essential role in the regulation of thrombus formation in the macrocirculation. AT localized to or in the vicinity of the vessel wall12 neutralizes the generated FIXa and restricts intrinsic Xase activity to the area of vascular injury. Similarly, in the microcirculatory cremaster injury model, FIX/FIXa-V16L generated thrombi over the entire experimental period, even at time points when FIX/FIXa-WT was inefficient. These differences were especially relevant for fibrin, which accumulated in higher amounts in the presence of FIX/FIXa-V16L than in FIX/FIXa-WT. This indicates that spatial and temporal regulation of FIXa activity by AT is essential for controlling thrombin production in small vascular beds.
In addition to the limitations imposed by FIXa inhibition, intrinsic Xase activity is restricted by FVIII/FVIIIa availability, which is regulated by von Willebrand factor, activation by thrombin, and inactivation by A2-domain dissociation and activated protein C.3,4,37 Previous studies suggest that the strength of the FIXa-FVIIIa interaction has a profound influence on clot formation as mutations that optimize the interaction increase clot formation and mutations that disrupt the interaction reduce clot formation.31,38 Therefore, we suggest a model where both cofactor- and protease-dependent mechanisms limit intrinsic Xase activity to the site of vessel injury, ultimately preventing unwanted thrombus formation. It is possible that the procoagulant effect of FIXa-V16L could also increase the lifetime of the intrinsic Xase complex. Because the variant is not inhibited well by AT, it could effectively preserve the activity of FVIIIa by limiting A2 domain dissociation by remaining in a complex with the cofactor.
Other inhibitors such as PS may also regulate FIXa activity. For example, mutations in FIXa heparin-binding exosite that affect PS-FIXa interaction increase thrombus formation in a venous injury model.39 Given that the FIXa-PS interaction involves both catalytic and EGF-1 domains we speculate that FIXa-V16L may also be resistant to PS inhibition and could contribute to the dominant procoagulant effect of this variant.
Our study also supports the idea that modifying FIXa inhibition may be advantageous in HB settings. Targeting AT function through a small interfering RNA strategy has proven to be successful in restoring hemostasis in both HA and HB.40 Findings of this study suggest that restricting FIXa inhibition may be advantageous, as it will result in a prolonged half-life in plasma for the protease and be functionally active when assembled in the intrinsic Xase complex. Furthermore, variants, such as FIX-V16L, could be advantageous in gene therapy settings to circumvent vector dose-dependent limitations.
In summary, our data demonstrate that the regulation of FIXa activity by plasma-based anticoagulants represents a critical control point to limit thrombus formation at the site of vascular injury in both macro- and microcirculation. Our findings not only provide mechanistic insights into the control of thrombus formation in vivo but also raise the possibility of modulating FIXa inhibition for therapeutic purposes.
Acknowledgments
The authors thank Sriram Krishnaswamy and Ben Samelson-Jones (Children’s Hospital of Philadelphia/University of Pennsylvania) for their critical review of this manuscript.
This work was supported in part by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (P01 HL139420, Project 4) (V.R.A. and R.M.C.), and an Early Career Development Grant from Bayer (L.I.).
Authorship
Contribution: L.I. conducted the experiment, analyzed the data, and wrote the manuscript; V.R.A. wrote the manuscript; R.M.C. analyzed the data and wrote the manuscript; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: R.M.C. is a consultant for Pfizer, Bayer, and Alnylam and receives research support from Bayer and Alnylam. The remaining authors declare no competing financial interests.
Valder R. Arruda died on 20 May 2022.
Correspondence: Lacramioara Ivanciu, Division of Hematology, The Children's Hospital of Philadelphia, 5022 Colket Translational Research Building, 3501 Civic Center Blvd, Philadelphia, PA, 19104; e-mail: ivanciul@chop.edu; and Rodney M. Camire, Division of Hematology, The Children's Hospital of Philadelphia, 5018 Colket Translational Research Building, 3501 Civic Center Blvd, Philadelphia, PA, 19104; e-mail: rcamire@pennmedicine.upenn.edu.
References
Author notes
Data are available on request from the corresponding authors, Lacramioara Ivanciu (ivanciul@chop.edu) and Rodney M. Camire (rcamire@pennmedicine.upenn.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.