In this issue of Blood, Elitzur et al1 reported that the Epstein-Barr virus (EBV) is associated with immune deficiency associated lymphoproliferative disorders (IA-LPD) in children treated for acute lymphoblastic leukemia (ALL). EBV is a family member of the gammaherpesvirus group, sometimes known as human herpesvirus 4 (HHV-4), and is relatively ubiquitous, infecting 80% to 95% of the human population.2 Immunocompetent hosts generally, especially young children, are asymptomatic during their primary EBV infection, although adolescents and young adults often have infectious mononucleosis symptoms with primary EBV infections.3
Unfortunately, EBV tends to persist lifelong (latency) in human hosts by residing in the memory B-cells. During periods of immunoincompetence (due to disease and/or immunosuppression), EBV reactivation and lytic replication may occur, sometimes resulting in EBV-associated malignancies. Previously reported EBV-associated malignancies include Burkitt lymphoma, NK/T-cell lymphoma, nasopharyngeal carcinoma, Hodgkin lymphoma, diffuse large B-cell lymphoma, gastric carcinoma, HIV-associated lymphomas, post-transplant lymphoproliferative disease, and IA-LPDs.2 The ability of EBV to evade both host innate and adaptive immunity though specific EBV proteins likely accounts for this malignant potential.4 Furthermore, we have previously described a distinctly different proteome between EBV-positive and EBV-negative Burkitt lymphoma.5 They are 2 subgroups of Burkitt lymphoma, EBV-positive and EBV-negative, with a predominance of EBV positivity in the African subtype and EBV negativity in Western Europe and North America subtypes.
In this issue of Blood, Elitzur et al report for several international groups ∼85 patients with childhood ALL that developed non-Hodgkin lymphoma (NHL), approximately two-thirds with mature B-cell lymphoproliferative disease, 26% with lymphoblastic lymphoma and the remainder minority with peripheral T-cell lymphoma or NHL NOS over a span of 38 years.1 Importantly, among these 85 cases, two-thirds demonstrated histological characteristics associated with immune deficiency associated lymphoproliferative diseases (IA-LPD) with predominant evidence of EBV driven lymphoproliferation.1 More than 80% of these cases occurred either during ALL maintenance chemotherapy or within 6 months following the end of maintenance therapy.1 Treatment varied but included stopping maintenance therapy, rituximab immunotherapy, rituximab plus low intensity chemotherapy or chemotherapy alone. Despite the majority of these patients presenting with stage III/IV disease, the outcome was somewhat better than that predicted with a probability of 5 years EFS and OS of 66.6% (95% CI, 55.3-80.1) and 67.4% (95% CI, 56-81), respectively and 5 years cumulative risk of mortality secondary to lymphoid neoplasm of 20.2% (95% CI, 10.2-30) and underlying leukemia mortality of 12.4% (95% CI, 2.8-22). Interestingly, in the multivariable analysis, only the presence of hemophagocytic lymphohistocytosis at diagnosis was the only variable associated with increased risk of mortality (HR, 7.2; 95% CI, 1.62-32.98) (P = .01).1
This report represents the largest series of these cases to date. The authors should be congratulated on the extensive analyses they performed through the Ponte di Legno childhood ALL consortium that included 12 international consortia, the international surveys performed through the International BFM Study Group (I-BFM) and St Jude Global, and extensive literature searches to identify and classify all cases. Although the authors suggest that maintenance therapy during childhood ALL treatment resulted in an “immunocompetent state” thereby creating an immunodeficient environment that facilitated EBV reactivation and EBV-associated oncogenesis, they were unfortunately unable to investigate the specific immunodeficiencies associated with each patient to confirm this hypothesis. Despite this limitation, this observation is highly suggestive of a secondary immunodeficient state during routine maintenance childhood ALL treatment, which predisposes a small group of patients to develop EBV-associated IA-LPD. One hypothesis that is not fully discussed is whether some of the patients may have had a subtle primary immunodeficiency, which contributed to the development of both primary ALL and secondary IA-LPD. In the future, we will have tools to easily diagnose subtle forms of primary immunodeficiency at the time of diagnosis in all children with ALL to determine whether this is the causative mechanism.
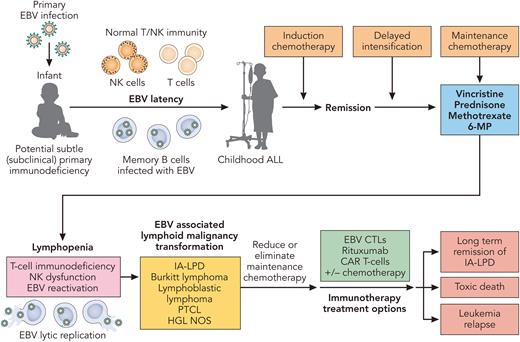
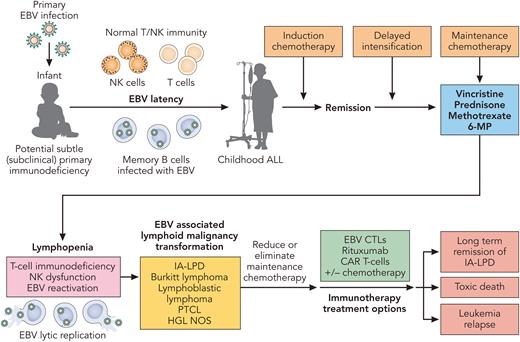
What are the potential mechanisms responsible for these astute observations. As the authors pointed out in their discussion, both methotrexate and thiopurines (6-MP) have previously been associated with IA-LPDs.6,7 Other potential mechanisms include chemotherapy induced lymphopenia, T-cell immunodysfunction, and/or chemotherapy associated innate immunodeficiency (see figure).8 As with most EBV-associated IA-LPD, rapid restoration of EBV-specific T-cell immunity is critical to eradicating these EBV-associated LPDs. Recently, we and others have investigated the use of EBV-specific adoptive T-cell therapy with either third-party (HLA ≥ 1 match) cytotoxic T cells manufactured by ex vivo expansion or second-party/haploidentical EBV-specific cytotoxic T-cell lymphocytes obtained by enriching familial haploidentical memory EBV-CTLs utilizing the Cytokine Capture System in patients with persistent EBV-associated infections and T-cell immunodeficiency.9,10 These new cellular therapy approaches to boost and rapidly restore EBV T-cell immunity may become more universally available in the future for the treatment of these patients who have already been heavily treated. Lastly, how much ALL maintenance therapy is really needed? Are there subsets of children with ALL who are genetically predisposed to developing IA-LPDs, where less maintenance may be more beneficial in the long run? As with most thought provoking studies, such as this one, sometimes the results precipitate more questions than answers and require future investigations.
Potential mechanisms and treatment of EBV-associated immune deficiency associated- lymphoproliferative disorders (IA-LPD) in children treated for acute lymphoblastic leukemia. Professional illustration by Patrick Lane, ScEYEnce Studios.
Potential mechanisms and treatment of EBV-associated immune deficiency associated- lymphoproliferative disorders (IA-LPD) in children treated for acute lymphoblastic leukemia. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conflict-of-interest disclosure: M.S.C. has served as a consultant for Jazz Pharmaceuticals, Omeros Pharmaceuticals, Servier Pharmaceuticals, NEKTAR and Novartis Pharmaceuticals; been a member of the speakers’ bureaus for Jazz Pharmaceuticals, Servier Pharmaceuticals, Amgen, Inc, Sanofi and Sobi; served on an advisory board for AstraZeneca; and received research funding from Celularity, Merck, Miltenyi Biotec, Servier, Omeros and Jazz. He has no conflicts of interest related to this publication.