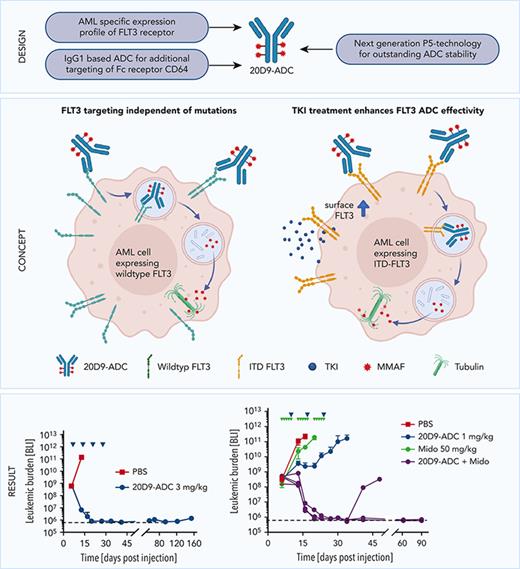
Key Points
FLT3 is a promising target for ADCs in AML therapy.
Combination with midostaurin enhances the effectivity of FLT3-ADC in FLT3-ITD–mutated AML.
Abstract
Fms-like tyrosine kinase 3 (FLT3) is often overexpressed or constitutively activated by internal tandem duplication (ITD) and tyrosine kinase domain (TKD) mutations in acute myeloid leukemia (AML). Despite the use of receptor tyrosine kinase inhibitors (TKI) in FLT3-ITD–positive AML, the prognosis of patients is still poor, and further improvement of therapy is required. Targeting FLT3 independent of mutations by antibody-drug conjugates (ADCs) is a promising strategy for AML therapy. Here, we report the development and preclinical characterization of a novel FLT3-targeting ADC, 20D9-ADC, which was generated by applying the innovative P5 conjugation technology. In vitro, 20D9-ADC mediated potent cytotoxicity to Ba/F3 cells expressing transgenic FLT3 or FLT3-ITD, to AML cell lines, and to FLT3-ITD–positive patient-derived xenograft AML cells. In vivo, 20D9-ADC treatment led to a significant tumor reduction and even durable complete remission in AML xenograft models. Furthermore, 20D9-ADC demonstrated no severe hematotoxicity in in vitro colony formation assays using concentrations that were cytotoxic in AML cell line treatment. The combination of 20D9-ADC with the TKI midostaurin showed strong synergy in vitro and in vivo, leading to reduction of aggressive AML cells below the detection limit. Our data indicate that targeting FLT3 with an advanced new-generation ADC is a promising and potent antileukemic strategy, especially when combined with FLT3-TKI in FLT3-ITD–positive AML.
Introduction
Acute myeloid leukemia (AML) is characterized by uncontrolled growth of differentiation arrested hematopoietic stem and progenitor cells. The 5-year survival rate in the United States is 29.5% (Surveillance, Epidemiology, and End Results Program data, 2011-2017), which shows the high medical need to improve therapy.1-5 Approaches to increase efficacy of the standard 7+3 chemotherapy include the combination with targeting agents such as receptor tyrosine kinase (RTK) inhibitors (TKIs) midostaurin and gilteritinib and gemtuzumab-ozogamicin (GO), an antibody-drug conjugate (ADC) against CD33.6,7 ADCs combine the specificity of antibodies with a highly potent drug,8,9 and the mechanism of action includes binding to the target, internalizing, and releasing the payload to kill the target cells.10 Currently, several ADCs are being investigated in preclinical settings for AML treatment, targeting, for example, C-lectin-like molecule 1, CD123, interleukin receptor α, or CXCR4 and fms-like tyrosine kinase 3 (FLT3).11-16 The latter is a member of the class III protein RTK and represents an established receptor for targeted therapies.17 The binding of the FLT3-ligand induces phosphorylation, internalization and activation of downstream targets involved in survival, and expansion of hematopoietic cells.18 In healthy tissue, FLT3 cell surface expression is restricted to granulocytes/macrophage progenitors, a subset of hematopoietic stem cells and differentiated monocytes, dendritic cells, and natural killer cells.18-21 Remarkably, FLT3 is expressed on blasts and leukemic stem cells of most AML patients. The expression levels are significantly higher compared with healthy tissue, and high levels of FLT3 were reported as risk factor in prognosis.22,23 Furthermore, activating internal tandem duplication (ITD) mutations are among the most frequent genetic abnormalities in AML and occur in ∼30% of patients at diagnosis. FLT3-ITD is associated with a high risk of relapse and a poor clinical outcome.24-27 FLT3-targeting agents, like midostaurin, are successfully applied in AML treatment.28
Here, we report the development of a novel mutation-independent FLT3-targeting ADC for AML treatment and provide proof of concept of its efficacy in preclinical in vitro and in vivo models. Recently, we have shown that the treatment of FLT3-ITD–mutated AML cells with the TKI quizartinib led to an increased FLT3 expression.29 Accordingly, a combination with FLT3-TKIs improved the efficacy of the FLT3 ADC both in vitro and in vivo.
Methods
Cell lines
Cell lines (supplemental Table 1, available on the Blood website) were cultured according to the supplier’s recommendations. For stable recombinant protein expression, Ba/F3 cells were retrovirally transduced as described before.30
Primary samples
Primary AML samples were obtained within trials AMLCG-99 (NCT00266136) and AMLCG-2008 (NCT01382147). Healthy bone marrow samples were obtained and isolated as described before.31 The study was performed in accordance with the ethical standards of the responsible committee on human experimentation (approval numbers LMU 068-08, LMU 222-10, and TUM 538/16) and with the Helsinki Declaration of 1975, as revised in 2013.
Binding and internalization of monoclonal antibodies
For antibody binding studies, cells were stained on ice with primary mouse, rat, or human anti-FLT3 antibodies (in-house) and secondary antibody goat F(ab')2 anti-human immunoglobulin-PE (Ig-PE) (2012-09), goat anti-mouse IgG(H+L)-PE (1032-09), or goat anti-rat IgG(H+L)-PE (3052-09) purchased from Southern Biotech. For internalization experiments, cells incubated with anti-FLT3 antibodies were washed and incubated for 30 minutes at 4°C or 37°C followed by staining with secondary antibody.
Cytotoxicity proliferation assays
Suspension cells were treated with 20D9-ADC or IgG1-ADC (in-house), deglycosylated ADC generated by applying Endo S (P0741L NEB), 20D9 monoclonal antibodies (mAb) (in-house), palivizumab (404770, AbbVie), quizartinib (S1526, Selleck Chem), or midostaurin (MedChemExpress). AML cells were treated once (day 0), and viability was determined after 96 hours using resazurin solution (50 μM final concentration, 4-hour incubation) (R12204, Thermo Fisher Scientific). For Ba/F3 cell assays, cells were treated once (day 0), and viable cells were counted after 72 hours on Vi-Cell Cell Viability Analyzer (Beckman Coulter, Krefeld, Germany). Calculation of 50% inhibitory concentration (IC50) values was performed using GraphPad Prism version 6.07 (GraphPad Software, La Jolla, CA).
In vivo experiments
Patient-derived xenograft (PDX) cells or MOLM-13 cells expressing enhanced firefly luciferase (luc) and mCherry (Addgene, plasmid #104833) were established as described previously.32 For in vivo therapy trials, luc+ MOLM-13 cells or luc− or luc+ PDX cells were injected intravenously (IV) into 8- to 12-week-old male NSG mice (NOD scid gamma, The Jackson Laboratory, Bar Harbor, ME), and tumor growth was regularly monitored by blood analysis or bioluminescence imaging (BLI).32 After successful engraftment, mice were treated with deglycosylated or native 20D9-ADC (1 or 3 mg/kg, IV, 1 dose per week), IgG1-ADC (3 mg/kg), or midostaurin (SelleckChem, 50 mg/kg, oral gavage, 5 doses per week; in 5% DMSO + 45% PEG300 + 50% ddH2O). Experimental endpoints were BLI values above 1 × 1010 photons/s or below detection limit (4 × 106 photons/s) for 90 to 150 days post injection, or blood values >45% hCD45+ hCD33+ cells. Mice showing clinical signs of illness or weight loss >15% under therapy were euthanized (1 ADC-treated mouse in Figure 4D). Mice that died in inhalation narcosis were excluded from further analysis (1 ADC-treated mouse in Figure 4D).
All animal trials were performed in accordance with the current ethical standards (Regierung von Oberbayern, number ROB-55.2Vet-2532.Vet_02-16-7).
Further information is provided in the supplemental Methods.
Results
Generation and characterization of anti-FLT3 antibodies
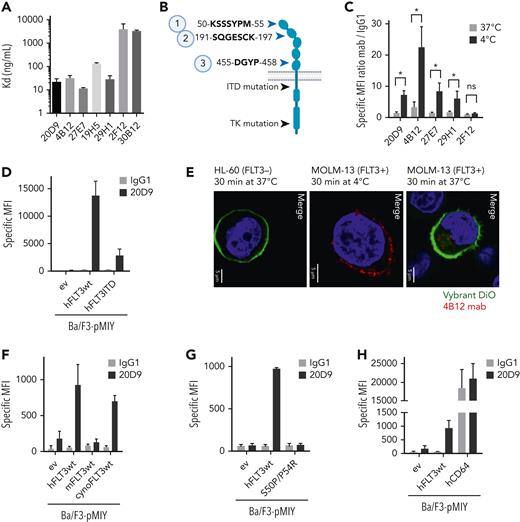
FLT3-specific mAbs were generated by hybridoma cells of isolated B cells from immunized rats and mice. After selection procedures, 7 antibodies were chimerized using a human IgG1 sequence (supplemental Figure 1A). This antibody scaffold maintains the ability to interact with Fc γ receptors (FcgRs), especially the high-affinity variant FcγR1 (CD64), which is also expressed on AML blasts and was already evaluated for targeted therapy.35-37 Chimeric antibodies were efficiently expressed in HEK293-F cells (supplemental Figure 1B) and possessed high protein stability (supplemental Figure 1C). Binding affinities to recombinant FLT3 protein varied from dissociation constant (KD) = 11.5 ng/mL to KD = 3981 ng/mL between the different clones (Figure 1A). Epitope mapping of the antibodies to peptides derived from extracellular domain of FLT3 identified 2 main binding motifs, KSSSYPM (bound by 30B12, 29H1, 27E7, 20D9) and SQGESCK (bound by 19H5, 4B12, 2F12) (Figure 1B, supplemental Figure 1D). The 20D9 showed additional affinity to a third minor epitope, DGYP.
Evaluation of epitope specificity and internalization capability of anti-FLT3 monoclonal antibodies. (A) Affinity of 7 anti-FLT3 mAb clones to recombinant human FLT3 measured in enzyme-linked immunosorbent assay normalized to the binding of the antibodies to bovine serum albumin control. KD (mean ± SD; n = 3) is depicted. (B) Schematic FLT3 receptor. Black arrows indicate common mutations, and blue arrows indicate the 3 identified epitopes of 7 anti-FLT3 antibodies analyzed in linear epitope mapping by PEPperPRINT. Figure was created with BioRender.com. (C) Binding and temperature-induced internalization of anti-FLT3 mAb in Ba/F3 cells expressing human wildtype FLT3 (hFLT3wt). Internalization was induced by incubation for 30 minutes at 37°C (gray) compared with 4°C (black). Remaining surface-bound antibody was detected in flow cytometry. Mean fluorescence intensity (MFI) was normalized to control human IgG1 binding. Mean ± SD of n = 3 is depicted. (D) Cell surface binding of 20D9-mAb or control human IgG1 antibody to Ba/F3 cells stably expressing pMIY (Ba/F3-pMIY) empty vector (ev), hFLT3wt, or ITD mutant human FLT3 (hFLT3ITD) was measured in flow cytometry. Mean ± SD of n = 3. (E) Temperature-induced internalization of 4B12-mAb in MOLM-13 (FLT3+) cells after 30-minutes incubation at 37°C compared with 4°C and HL-60 (FLT3−) after 37°C incubation assessed in immunofluorescence staining. Red = FLT3 staining by 4B12, green = membrane staining by Vybrant DiO, blue = nuclear staining by DAPI. Scale bar, 5 μm. Representative pictures are shown. (F) Cell surface binding of 20D9-mAb or control hIgG1 antibody to Ba/F3-pMIY ev, hFLT3wt, murine (mFLT3wt), or cynomolgues monkey FLT3 (cynoFLT3wt) was measured in flow cytometry. Mean ± SD of n = 3. (G) Cell surface binding of 20D9-mAb or control hIgG1 antibody to Ba/F3-pMIY ev, hFLT3wt, or epitope mutant FLT3 (hFLT3S50P/P54R) measured in flow cytometry. Mean ± SD of n = 3. (H) Cell surface binding of 20D9-mAb or control hIgG1 antibody to Ba/F3-pMIY ev, hFLT3wt, or human CD64 (hCD64) measured in flow cytometry. Mean ± SD of n = 3. ∗P < .05 (unpaired, 2-tailed t test). ns, not significant.
Evaluation of epitope specificity and internalization capability of anti-FLT3 monoclonal antibodies. (A) Affinity of 7 anti-FLT3 mAb clones to recombinant human FLT3 measured in enzyme-linked immunosorbent assay normalized to the binding of the antibodies to bovine serum albumin control. KD (mean ± SD; n = 3) is depicted. (B) Schematic FLT3 receptor. Black arrows indicate common mutations, and blue arrows indicate the 3 identified epitopes of 7 anti-FLT3 antibodies analyzed in linear epitope mapping by PEPperPRINT. Figure was created with BioRender.com. (C) Binding and temperature-induced internalization of anti-FLT3 mAb in Ba/F3 cells expressing human wildtype FLT3 (hFLT3wt). Internalization was induced by incubation for 30 minutes at 37°C (gray) compared with 4°C (black). Remaining surface-bound antibody was detected in flow cytometry. Mean fluorescence intensity (MFI) was normalized to control human IgG1 binding. Mean ± SD of n = 3 is depicted. (D) Cell surface binding of 20D9-mAb or control human IgG1 antibody to Ba/F3 cells stably expressing pMIY (Ba/F3-pMIY) empty vector (ev), hFLT3wt, or ITD mutant human FLT3 (hFLT3ITD) was measured in flow cytometry. Mean ± SD of n = 3. (E) Temperature-induced internalization of 4B12-mAb in MOLM-13 (FLT3+) cells after 30-minutes incubation at 37°C compared with 4°C and HL-60 (FLT3−) after 37°C incubation assessed in immunofluorescence staining. Red = FLT3 staining by 4B12, green = membrane staining by Vybrant DiO, blue = nuclear staining by DAPI. Scale bar, 5 μm. Representative pictures are shown. (F) Cell surface binding of 20D9-mAb or control hIgG1 antibody to Ba/F3-pMIY ev, hFLT3wt, murine (mFLT3wt), or cynomolgues monkey FLT3 (cynoFLT3wt) was measured in flow cytometry. Mean ± SD of n = 3. (G) Cell surface binding of 20D9-mAb or control hIgG1 antibody to Ba/F3-pMIY ev, hFLT3wt, or epitope mutant FLT3 (hFLT3S50P/P54R) measured in flow cytometry. Mean ± SD of n = 3. (H) Cell surface binding of 20D9-mAb or control hIgG1 antibody to Ba/F3-pMIY ev, hFLT3wt, or human CD64 (hCD64) measured in flow cytometry. Mean ± SD of n = 3. ∗P < .05 (unpaired, 2-tailed t test). ns, not significant.
To identify their suitability for ADC development, the binding and internalization efficiencies of the antibodies to Ba/F3 cells stably expressing human wildtype FLT3 (hFLT3wt) or empty MSCV-IRES-YFP vector (pMIY ev) were evaluated. The clones 20D9, 4B12, 29H1, and 27E7 specifically bound the Ba/F3-hFLT3wt cells as analyzed by flow cytometry (Figure 1C and negative control in supplemental Figure 1E), and 20D9 showed specific binding to Ba/F3 cells expressing hFLT3ITD (Figure 1D, receptor expression in supplemental Figure 1F) and/or TKD-mutated FLT3 (supplemental Figure 1G, receptor expression in supplemental Figure 1H, supplemental Results). Further, the antibodies displayed significant internalization of ∼80% in flow cytometry-based internalization assays in the Ba/F3-hFLT3wt cells (Figure 1C, supplemental Figure 2A). These observations could be confirmed in FLT3+ AML cell lines in flow cytometry and immunofluorescence staining (Figure 1E, supplemental Figure 2B-C). Furthermore, the internalized antibodies were localized to early endosomes, which was demonstrated by the co-localization with EEA1 (supplemental Figure 2D).
Based on internalization and high expression yields, we selected the 20D9 clone for further development and evaluated the binding to FLT3 orthologs from different species. The protein sequence of human FLT3 in epitope 1 is identical to the cynoFLT3 and differs from the mFLT3 receptor (supplemental Figure 3A). In contrast to the mFLT3, the cynoFLT3 expressing Ba/F3 cells bound the 20D9-mAb (Figure 1F, receptor expression in supplemental Figure 3B-C). To proof the epitope specificity, we expressed a human FLT3 receptor with the epitope region mutated to the murine variant (FLT3S50P/P54R) in Ba/F3 cells (supplemental Figure 3D), which did not bind the 20D9-mAb (Figure 1G, supplemental Figure 3E). Finally, we verified the binding of 20D9-mAb to the high-affinity Fc receptor CD64 via the IgG1 backbone using Ba/F3 cells expressing human CD64 (Figure 1H, supplemental Figure 3F).
Generation and characterization of 20D9-ADC
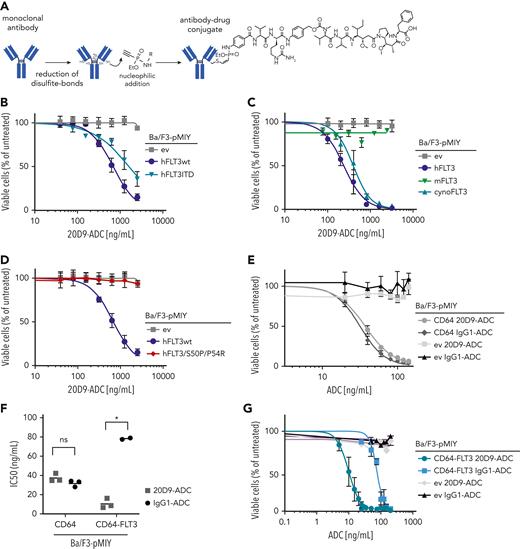
We applied the P5 technology, which uses ethynylphosphonamidates for a stable conjugation to the antibodies’ cysteine residues.38,39 We conjugated IgG1-based 20D9 with the tubulin polymerization inhibitor monomethyl auristatin F (MMAF) payload with a drug-to-antibody ratio (DAR) of 6.2 (Figure 2A). Incubation for 2 weeks at 40°C or storage for 14 months at 4°C did not reduce the antibody-toxin conjunction (supplemental Figure 4A-B) and only slightly induced the aggregation (supplemental Figure 4C-D). Furthermore, to obtain an IgG1-ADC only possessing the CD64 but no FLT3 binding, we conjugated MMAF to the IgG1-based antibody palivizumab, which is specific for the glycoprotein F of the respiratory syncytial virus.40
Analysis of cytotoxicity of FLT3-specific 20D9-ADC to different FLT3 variants. (A) Schematic process of P5 conjugation technology39 via disulfite bond reduction and Staudinger-induced Michael addition. Final ADC consists of monoclonal antibody (20D9 or IgG1 antibody) coupled to monomethyl auristatin F toxin. Figure was created with BioRender.com. (B-E,G) Assessment of cytotoxicity of ADCs in different Ba/F3 cell lines. Viability was determined after 72-hour treatment with different ADC concentrations by trypan blue exclusion count and compared with untreated control. Mean ± SD of n = 3 biological replicates. (B) Treatment of Ba/F3-pMIY ev, hFLT3wt, and hFLT3ITD cells with 20D9-ADC. (C) Treatment of Ba/F3-pMIY ev, hFLT3wt, mFLT3wt, and cynoFLT3wt cells with 20D9-ADC. (D) Treatment of Ba/F3-pMIY ev, hFLT3wt, and hFLT3S50P/P54R cells with 20D9-ADC. (E) Treatment of Ba/F3-pMIY ev and hCD64 with either 20D9-ADC or IgG1-ADC. (F) IC50 values of 20D9-ADC and IgG1-ADC in Ba/F3-pMIY hCD64 and hCD64-hFLT3 cells. Calculation based on data from Figure 2E and G calculated by GraphPad Prism. Each dot represents a replicate; horizontal line indicates mean. (G) Treatment of Ba/F3-pMIY ev and hCD64-hFLT3 with different concentrations of either 20D9-ADC or IgG1-ADC. ∗P < .05 (unpaired, 2-tailed t test). ns, not significant.
Analysis of cytotoxicity of FLT3-specific 20D9-ADC to different FLT3 variants. (A) Schematic process of P5 conjugation technology39 via disulfite bond reduction and Staudinger-induced Michael addition. Final ADC consists of monoclonal antibody (20D9 or IgG1 antibody) coupled to monomethyl auristatin F toxin. Figure was created with BioRender.com. (B-E,G) Assessment of cytotoxicity of ADCs in different Ba/F3 cell lines. Viability was determined after 72-hour treatment with different ADC concentrations by trypan blue exclusion count and compared with untreated control. Mean ± SD of n = 3 biological replicates. (B) Treatment of Ba/F3-pMIY ev, hFLT3wt, and hFLT3ITD cells with 20D9-ADC. (C) Treatment of Ba/F3-pMIY ev, hFLT3wt, mFLT3wt, and cynoFLT3wt cells with 20D9-ADC. (D) Treatment of Ba/F3-pMIY ev, hFLT3wt, and hFLT3S50P/P54R cells with 20D9-ADC. (E) Treatment of Ba/F3-pMIY ev and hCD64 with either 20D9-ADC or IgG1-ADC. (F) IC50 values of 20D9-ADC and IgG1-ADC in Ba/F3-pMIY hCD64 and hCD64-hFLT3 cells. Calculation based on data from Figure 2E and G calculated by GraphPad Prism. Each dot represents a replicate; horizontal line indicates mean. (G) Treatment of Ba/F3-pMIY ev and hCD64-hFLT3 with different concentrations of either 20D9-ADC or IgG1-ADC. ∗P < .05 (unpaired, 2-tailed t test). ns, not significant.
In cytotoxicity assays, hFLT3wt, hFLT3ITD, and hFLT3TKD mutants (Figure 2B, supplemental Figure 4E-G; supplemental Results) as well as cynoFLT3 (Figure 2C, supplemental Figure 4H) expressing Ba/F3 cells were sensitive to 20D9-ADC treatment. Consistent with the binding analysis, 20D9-ADC was not cytotoxic in Ba/F3-mFLT3 or epitope mutant Ba/F3-hFLT3S50P/P54R cells (Figure 2C-D). As controls we tested the cytotoxicity of MMAF and control IgG1-ADC in Ba/F3 cells expressing the empty vector or hFLT3wt. MMAF killed Ba/F3 cells only at high concentrations and independent of FLT3 expression, whereas IgG1-ADC had no effect on Ba/F3 cell viability at all (supplemental Figure 4I-J).
Furthermore, we assessed the cytotoxicity mediated by the IgG1-FcγR binding of the 20D9-ADC. Ba/F3 cells expressing hCD64 were sensitive to 20D9-ADC and IgG1-ADC with similar mean IC50 of 37.3 ng/mL and 31.8 ng/mL, respectively (Figure 2E-F), whereas Ba/F3 cells expressing hCD16 or hCD32 did not respond to 20D9-ADC (supplemental Figure 4K). Ba/F3 cells expressing both hFLT3wt and hCD64 (supplemental Figure 4L) were significantly more sensitive to 20D9-ADC compared with IgG1-ADC (IC50 = 0.5 ng/mL vs 78.3 ng/mL), indicating the advantage of targeting both antigens in vitro (Figure 2F-G).
In vitro cytotoxic activity of 20D9-ADC in AML cell lines
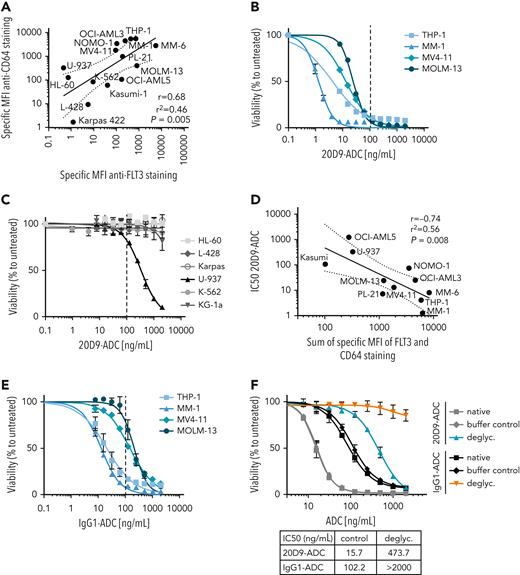
We determined the expression levels of FLT3 and CD64 in different leukemia and lymphoma cell lines (supplemental Figure 5A-B) and could detect a significant correlation (Figure 3A). Binding of the 20D9 antibody (supplemental Figure 5C) and cytotoxicity of the 20D9-ADC could be shown in all FLT3+ cell lines with IC50 values varying between 1.3 ng/mL and 107.33 ng/mL (Figure 3B, supplemental Table 1). Furthermore, a TP53 knockdown in FLT3+ AML cell lines only slightly altered the IC50 of 20D9-ADC and FLT3 expression (supplemental Figure 5D-F), indicating that a TP53 mutation is not likely to compromise efficacy.
Analysis of 20D9-ADC and control IgG1-ADC cytotoxicity in leukemia and lymphoma cell lines. (A) Correlation of MFI of CD64 cell surface expression and MFI of FLT3 cell surface expression of myeloid human cell lines measure in flow cytometry. Expression data presented in supplemental Figure 5A-B. Black lines indicates simple linear regression with error interval. R = Pearson correlation coefficient; r2 = coefficient of determination; P value from 2-tailed test with confidence interval of 95%. (B,C,E,F) Assessment of cytotoxicity of ADCs in different human cell lines. Viability was determined after 96-hour treatment with different concentrations of ADCs by resazurin fluorescence and normalized to untreated control. Dashed line indicates 100 ng/mL drug concentration. Mean ± SD of n = 3 biological replicates. (B) Treatment of FLT3+ human cell lines with 20D9-ADC. (C) Treatment of FLT3− human cell lines with 20D9-ADC. (D) Correlation of IC50 values of 20D9-ADC and sum of MFI of FLT3 and CD64 cell surface expression of myeloid human cell lines measured in flow cytometry. Expression data presented in supplemental Figure 5A-B and IC50 values of 20D9-ADC in supplemental Table 1. Black lines indicates simple linear regression with error interval. (E) Treatment of FLT3+ human cell lines with IgG1-ADC. (F) Treatment of MOLM-13 cells with either native, buffer-incubated control (buffer control) or deglycosylated (deglyc.) 20D9-ADC or IgG1-ADC.
Analysis of 20D9-ADC and control IgG1-ADC cytotoxicity in leukemia and lymphoma cell lines. (A) Correlation of MFI of CD64 cell surface expression and MFI of FLT3 cell surface expression of myeloid human cell lines measure in flow cytometry. Expression data presented in supplemental Figure 5A-B. Black lines indicates simple linear regression with error interval. R = Pearson correlation coefficient; r2 = coefficient of determination; P value from 2-tailed test with confidence interval of 95%. (B,C,E,F) Assessment of cytotoxicity of ADCs in different human cell lines. Viability was determined after 96-hour treatment with different concentrations of ADCs by resazurin fluorescence and normalized to untreated control. Dashed line indicates 100 ng/mL drug concentration. Mean ± SD of n = 3 biological replicates. (B) Treatment of FLT3+ human cell lines with 20D9-ADC. (C) Treatment of FLT3− human cell lines with 20D9-ADC. (D) Correlation of IC50 values of 20D9-ADC and sum of MFI of FLT3 and CD64 cell surface expression of myeloid human cell lines measured in flow cytometry. Expression data presented in supplemental Figure 5A-B and IC50 values of 20D9-ADC in supplemental Table 1. Black lines indicates simple linear regression with error interval. (E) Treatment of FLT3+ human cell lines with IgG1-ADC. (F) Treatment of MOLM-13 cells with either native, buffer-incubated control (buffer control) or deglycosylated (deglyc.) 20D9-ADC or IgG1-ADC.
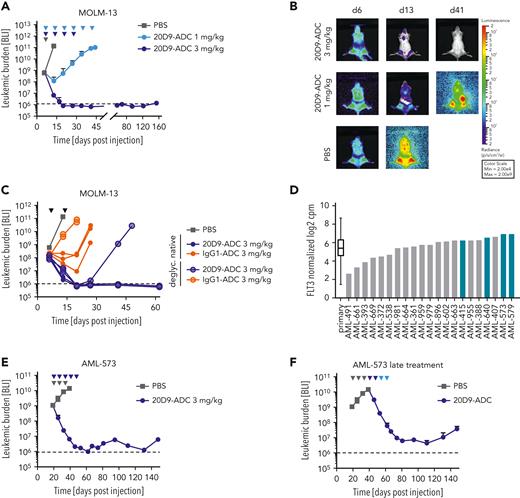
Evaluation of in vivo activity of 20D9-ADC in xenograft mouse models. NSG mice were injected IV with 1 × 105 luciferase expressing MOLM-13 cells (A-C) or 2 × 106 luciferase expressing AML-573 PDX cells (E-F). Leukemic burden was monitored once or twice a week by BLI, and total flux was quantified. Mean ± SD is depicted. Treatment is indicated with triangles in dark blue (20D9-ADC, 3 mg/kg), light blue (20D9-ADC, 1 mg/kg), gray (PBS), or black (all groups as indicated). Dashed black line indicates imaging threshold. (A) One week after transplantation, mice were treated with 20D9-ADC (1 mg/kg or 3 mg/kg, IV) or PBS as control (n = 4/group) once a week for 6 weeks (1 mg/kg) or for 4 weeks (3 mg/kg). (B) BLI pictures of 1 representative mouse per group are shown. (C) One week after transplantation, mice were treated with either native or glycosylated 20D9-ADC or with either native or glycosylated IgG1-ADC (3 mg/kg; n = 3/group) once a week for 2 weeks. PBS control mice of experiment shown in (A) are included as control. (D) FLT3 RNA expression of AML PDX samples (n = 21) and primary patient samples33 (n = 261) analyzed by single cell RNA barcoding sequencing.34 Data presented as normalized log2 counts per million. Samples selected for ex vivo and in vivo analysis are marked in blue. (E) 20 days after transplantation, mice were treated at intermediate tumor burden with 20D9-ADC (3 mg/kg) or PBS as control (n = 3/group) once a week for up to 4 weeks (2 mice) or 5 weeks (1 mouse). (F) PBS-treated control mice from (E) (n = 3) were treated at day 41 after transplantation at advanced tumor burden with 20D9-ADC once a week for 4 weeks (2 doses of 3 mg/kg, followed by 2 doses of 1 mg/kg).
Evaluation of in vivo activity of 20D9-ADC in xenograft mouse models. NSG mice were injected IV with 1 × 105 luciferase expressing MOLM-13 cells (A-C) or 2 × 106 luciferase expressing AML-573 PDX cells (E-F). Leukemic burden was monitored once or twice a week by BLI, and total flux was quantified. Mean ± SD is depicted. Treatment is indicated with triangles in dark blue (20D9-ADC, 3 mg/kg), light blue (20D9-ADC, 1 mg/kg), gray (PBS), or black (all groups as indicated). Dashed black line indicates imaging threshold. (A) One week after transplantation, mice were treated with 20D9-ADC (1 mg/kg or 3 mg/kg, IV) or PBS as control (n = 4/group) once a week for 6 weeks (1 mg/kg) or for 4 weeks (3 mg/kg). (B) BLI pictures of 1 representative mouse per group are shown. (C) One week after transplantation, mice were treated with either native or glycosylated 20D9-ADC or with either native or glycosylated IgG1-ADC (3 mg/kg; n = 3/group) once a week for 2 weeks. PBS control mice of experiment shown in (A) are included as control. (D) FLT3 RNA expression of AML PDX samples (n = 21) and primary patient samples33 (n = 261) analyzed by single cell RNA barcoding sequencing.34 Data presented as normalized log2 counts per million. Samples selected for ex vivo and in vivo analysis are marked in blue. (E) 20 days after transplantation, mice were treated at intermediate tumor burden with 20D9-ADC (3 mg/kg) or PBS as control (n = 3/group) once a week for up to 4 weeks (2 mice) or 5 weeks (1 mouse). (F) PBS-treated control mice from (E) (n = 3) were treated at day 41 after transplantation at advanced tumor burden with 20D9-ADC once a week for 4 weeks (2 doses of 3 mg/kg, followed by 2 doses of 1 mg/kg).
The 20D9-ADC acts via apoptosis induction, which was demonstrated in FLT3-ITD–positive MOLM-13 cells compared with FLT3− HL-60 cells (supplemental Figure 5G). Furthermore, we observed no cytotoxicity of 20D9-ADC to 5 out of 6 FLT3− AML cell lines. The FLT3−, CD64+ cell line U-937 showed an IC50 of 334 ng/mL (Figure 3C). Consistently, there was a correlation of summarized CD64 and FLT3 expression and the 20D9-ADC IC50 (Figure 3D).
The IgG1-ADC showed cytotoxic activity in all CD64+ cell lines (Figure 3E). The IC50 ranged from 12.82 to ∼2000 ng/mL and were noticeably higher compared with the 20D9-ADC (supplemental Table 1). FLT3+ and FLT3− cell lines showed similar sensitivity towards the payload MMAF (supplemental Figure 5H). The native 20D9 antibody or the IgG1 antibody did not impair cell proliferation (supplemental Figure 5I and data not shown).
To investigate the impact of CD64 interaction on the efficacy of IgG1-based ADCs, we disrupted the CD64-IgG1 binding by removing the N-linked glycans of 20D9-ADC and IgG1-ADC (supplemental Figure 5J). Compared with the native 20D9-ADC, the IC50 of deglycosylated 20D9-ADC shifted from 15.7 ng/mL to 473.7 ng/mL, reflecting the proportions of FLT3- and CD64-specific targeting. As control, the deglycosylated IgG1-ADC showed no activity on MOLM-13 cells, confirming the effective abrogation of the CD64-FcgR interaction (Figure 3F).
Antileukemic activity of 20D9-ADC in cell line and patient-derived xenograft AML mouse models
To determine the in vivo antileukemic activity of 20D9-ADC, we transplanted MOLM-13 or PDX cells into NSG mice. For sensitive monitoring of tumor burden by BLI, luc-expressing cells were used, which showed similar FLT3 expression levels compared with parental cells (supplemental Figure 6A).
First, we analyzed the efficacy of ADCs on MOLM-13 cells in vivo. Whereas repetitive administration of 1 mg/kg (once weekly ×6) 20D9-ADC decelerated the increase of tumor burden compared with phosphate-buffered saline (PBS)-treated mice, 3 mg/kg (once weekly × 4) led to a strong reduction of tumor burden below detection limit for at least 154 days (Figure 4A-B). The effect was comparable if therapy started at intermediate or advanced tumor burden (supplemental Figure 6B). To define CD64-related effects, we applied native and deglycosylated IgG1-ADC and 20D9-ADC. Interestingly, the deglycosylated 20D9-ADC showed strong cytotoxicity comparable with the native 20D9-ADC, indicating that FLT3 targeting is sufficient to elicit a long-lasting response. In contrast, the effect of native IgG1-ADC (3 mg/kg; once weekly ×2) was reduced compared with native 20D9-ADC, underlining that CD64 targeting was less effective in vivo. The deglycosylated IgG1-ADC had only a minimal effect compared with PBS treatment, confirming the functional abrogation of CD64-IgG1 interaction (Figure 4C).
Next, we determined the effect of 20D9-ADC on native and luc+ PDX samples.32 We selected samples with FLT3-ITD mutation and moderate to high FLT3 expression compared with AML patient samples33 (Figure 4D, supplemental Table 2). Ex vivo, the PDX cells were sensitive to 20D9-ADC treatment but not to 20D9-mAb treatment (supplemental Figure 6C-D). In vivo treatment of AML-573–transplanted mice with 3 mg/kg 20D9-ADC (once weekly ×5) led to a strong tumor reduction followed by stable low tumor burden up to 150 days, both if treatment started at intermediate or at advanced tumor burden (Figure 4E-F). The strong effect of 20D9-ADC could also be seen when native primograft AML-573 cells were transplanted (supplemental Figure 6E). Similarly, in 2 additional PDX samples, AML-640 and AML-579, treatment with 3 mg/kg 20D9-ADC (once weekly ×3) at intermediate or advanced tumor burden led to a strong tumor reduction followed by a tumor outgrowth after treatment stop (supplemental Figure 6F-I).
Hematotoxicity of 20D9-ADC
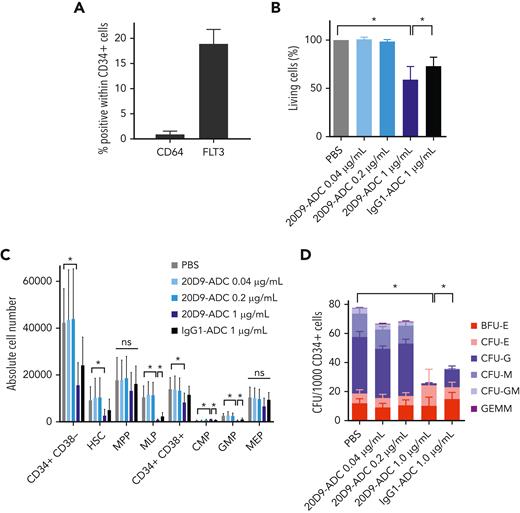
To assess hematotoxicity, we investigated the effect of 20D9-ADC on normal human hematopoietic cells in vitro by analyzing bone marrow cells from healthy donors enriched for CD34+ cells (supplemental Figure 7A-B). As expected, FLT3 expression could be detected in 18.9% ± 2.9% of CD34+ cells, whereas CD64 was barely expressed (1% ± 0.6%, Figure 5A). Cells were treated with ADC concentrations within the range of the observed IC50 in AML cells (40 and 200 ng/mL) and with high dose (1000 ng/mL). Only treatment with the high dose of both 20D9-ADC and IgG1-ADC led to a significant decrease in cell viability (Figure 5B), and this might indicate IgG1-dependent toxicity that induces a significant reduction of CD64-expressing cells after treatment (supplemental Figure 7C). Accordingly, an effect on the differentiation capacity could only be seen after treatment with high-dose 20D9-ADC, which revealed a significantly decreased proportion of hematopoietic stem cells, CD34+ CD38−, CD34+ CD38−, MLP, CMP, and GMP cell populations (Figure 5C). After treatment with IgG1-ADC, we observed significantly decreased MLPs, CMPs, and GMPs but to a lower extent compared with the 20D9-ADC. Furthermore, we assessed clonogenic capacity by colony-forming unit assay of healthy CD34+ cells (Figure 5D, supplemental Figure 7D). Again, only high-dose treatment with 20D9-ADC and IgG1-ADC revealed significantly reduced granulocytic, monocytic, and granulocytic-macrophagic colony formation. The erythroid progenitors were unaffected.
Analysis of hematotoxicity of 20D9-ADC. (A) Expression of FLT3 and CD64 in CD34+ healthy bone marrow (BM) cells measured in flow cytometry. Mean ± SD of n = 3 donors. (B-C) CD34+ cells were treated with 0.04 μg/mL, 0.2 μg/mL, or 1 μg/mL 20D9-ADC; 1 μg/mL IgG1-ADC; or PBS and analyzed in flow cytometry after 4 days. Kruskal-Wallis test; mean ± SD of n = 5. (B) Percentage of living cells measured with Annexin V/PI staining and normalized to PBS. (C) Differentiation assessment after staining to differentiation markers. CMP, common myeloid progenitors; GMP, granulocyte-monocyte progenitors; MEP, megakaryocyte/erythroid progenitors; MLP, multilymphoid progenitors; MPP, multipotent progenitors; HSC, hematopoietic stem cells. (D) Assessment of clonogenic capacity of healthy CD34+ BM cells. Cells were treated with 0.04 μg/mL, 0.2 μg/mL, or 1 μg/mL 20D9-ADC; 1 μg/mL IgG1-ADC; or PBS and plated afterwards for colony-forming unit (CFU) assay without further treatment. After 14 days, colonies were counted. GEMM, granulocyte, erythrocyte, macrophage, megakaryocyte; GM, granulocyte, macrophage; M, macrophage; G, granulocyte; E, erythrocyte; BFU-E, burst-forming unit erythrocyte. 2-way analysis of variance; mean ± SD, n = 5. ∗P < .05. ns, not significant.
Analysis of hematotoxicity of 20D9-ADC. (A) Expression of FLT3 and CD64 in CD34+ healthy bone marrow (BM) cells measured in flow cytometry. Mean ± SD of n = 3 donors. (B-C) CD34+ cells were treated with 0.04 μg/mL, 0.2 μg/mL, or 1 μg/mL 20D9-ADC; 1 μg/mL IgG1-ADC; or PBS and analyzed in flow cytometry after 4 days. Kruskal-Wallis test; mean ± SD of n = 5. (B) Percentage of living cells measured with Annexin V/PI staining and normalized to PBS. (C) Differentiation assessment after staining to differentiation markers. CMP, common myeloid progenitors; GMP, granulocyte-monocyte progenitors; MEP, megakaryocyte/erythroid progenitors; MLP, multilymphoid progenitors; MPP, multipotent progenitors; HSC, hematopoietic stem cells. (D) Assessment of clonogenic capacity of healthy CD34+ BM cells. Cells were treated with 0.04 μg/mL, 0.2 μg/mL, or 1 μg/mL 20D9-ADC; 1 μg/mL IgG1-ADC; or PBS and plated afterwards for colony-forming unit (CFU) assay without further treatment. After 14 days, colonies were counted. GEMM, granulocyte, erythrocyte, macrophage, megakaryocyte; GM, granulocyte, macrophage; M, macrophage; G, granulocyte; E, erythrocyte; BFU-E, burst-forming unit erythrocyte. 2-way analysis of variance; mean ± SD, n = 5. ∗P < .05. ns, not significant.
Treatment combination of 20D9-ADC and TKIs
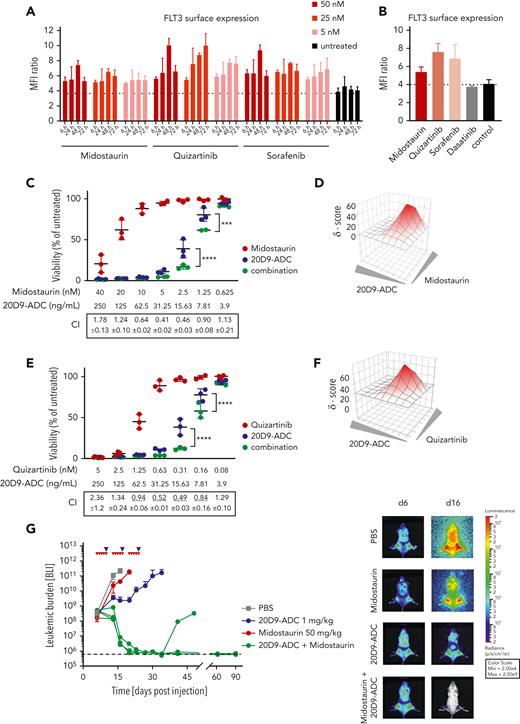
We have previously shown that TKI treatment increased the surface expression of FLT3 on FLT3-ITD–positive AML cells and sensitized them to bispecific FLT3 x CD3 antibodies.29 Thus, we combined the 20D9-ADC with TKIs for treatment of FLT3-mutated AML to increase the cytotoxic activity of the ADC. Incubation of MOLM-13 (FLT3-ITD heterozygote), MV4-11 (FLT3-LOH-ITD), and MM-6 (FLT3V592A) cells with midostaurin, quizartinib, or sorafenib led to an increase of FLT3 cell surface expression after incubation (Figure 6A-B and supplemental Figure 8A-B). To investigate whether this is a FLT3-TKI–specific effect, we determined the FLT3 expression as response to the control TKI dasatinib that targets BCR-ABL, c-KIT, EPH, and PDGFβ and is used in chronic myeloid leukemia, acute lymphoblastic leukemia, and AML therapy.41 Even though dasatinib exhibits similar effects on cell viability compared with FLT3-TKIs in selected concentrations (data not shown), dasatinib did not upregulate FLT3 surface expression (Figure 6B).
Treatment combination of 20D9-ADC and TKIs. (A-B) Upregulation of FLT3 cell surface expression in MOLM-13 cells after treatment with kinase inhibitors compared with untreated control. Cells were analyzed in flow cytometry after treatment. Dotted line represents MFI ratio of untreated cells. Mean ± SD of n = 2 is depicted. (A) Cells were treated with 5, 25, or 50 nM midostaurin, quizartinib, and sorafenib for 6, 24, 48, or 72 hours. (B) Cells were treated with 5 nM midostaurin, 1 nM quizartinib, 5 nM sorafenib, or 1 μM dasatinib for 72 hours. (C-F) Treatment combination of 20D9-ADC and midostaurin (C-D) or quizartinib (E-F) in MOLM-13 cells compared with treatment with 20D9-ADC, midostaurin, or quizartinib as single agent. Viability was determined after 96 hours by resazurin fluorescence and normalized to dimethyl sulfoxide−treated control. (C,E) Each dot represents 1 biological replicate; the horizontal line indicates the mean. Combination indices (CIs) with standard deviation were determined using CompuSyn software; CI < 1 indicates synergy and is underlined; CI = 1 additivity; CI > 1 antagonism. (D-F) The synergy score was calculated by Synergy Finder software using zero interaction potency (ZIP) modeling. Gray triangles indicate increasing drug concentrations. A positive Synergy score value δ and the red coloring indicate synergism. (G) Treatment combination of 20D9-ADC and midostaurin in vivo. NSG mice were injected IV with 1e5 luciferase-expressing MOLM-13 cells. Leukemic burden was monitored once or twice a week by BLI, and total flux was quantified (left). Mean ± SD is depicted. Dashed black line indicates imaging threshold. One week after transplantation, mice were treated for 3 weeks with 20D9-ADC (1 mg/kg IV, once per week), midostaurin (50 mg/kg by mouth, 5 days per week), a combination of both, or PBS as control (n = 4/group). Bioluminescence imaging of 1 representative mouse of each group at days 6 and 16 (right). Two-way analysis of variance: ∗∗∗P < .001; ∗∗∗∗P < .0001.
Treatment combination of 20D9-ADC and TKIs. (A-B) Upregulation of FLT3 cell surface expression in MOLM-13 cells after treatment with kinase inhibitors compared with untreated control. Cells were analyzed in flow cytometry after treatment. Dotted line represents MFI ratio of untreated cells. Mean ± SD of n = 2 is depicted. (A) Cells were treated with 5, 25, or 50 nM midostaurin, quizartinib, and sorafenib for 6, 24, 48, or 72 hours. (B) Cells were treated with 5 nM midostaurin, 1 nM quizartinib, 5 nM sorafenib, or 1 μM dasatinib for 72 hours. (C-F) Treatment combination of 20D9-ADC and midostaurin (C-D) or quizartinib (E-F) in MOLM-13 cells compared with treatment with 20D9-ADC, midostaurin, or quizartinib as single agent. Viability was determined after 96 hours by resazurin fluorescence and normalized to dimethyl sulfoxide−treated control. (C,E) Each dot represents 1 biological replicate; the horizontal line indicates the mean. Combination indices (CIs) with standard deviation were determined using CompuSyn software; CI < 1 indicates synergy and is underlined; CI = 1 additivity; CI > 1 antagonism. (D-F) The synergy score was calculated by Synergy Finder software using zero interaction potency (ZIP) modeling. Gray triangles indicate increasing drug concentrations. A positive Synergy score value δ and the red coloring indicate synergism. (G) Treatment combination of 20D9-ADC and midostaurin in vivo. NSG mice were injected IV with 1e5 luciferase-expressing MOLM-13 cells. Leukemic burden was monitored once or twice a week by BLI, and total flux was quantified (left). Mean ± SD is depicted. Dashed black line indicates imaging threshold. One week after transplantation, mice were treated for 3 weeks with 20D9-ADC (1 mg/kg IV, once per week), midostaurin (50 mg/kg by mouth, 5 days per week), a combination of both, or PBS as control (n = 4/group). Bioluminescence imaging of 1 representative mouse of each group at days 6 and 16 (right). Two-way analysis of variance: ∗∗∗P < .001; ∗∗∗∗P < .0001.
Different dose combinations of 20D9-ADC and TKI were applied to MOLM-13 cells in vitro. Although midostaurin as single drug did not affect cell viability at low doses, combination treatment with the 20D9-ADC was significantly beneficial compared with 20D9-ADC treatment alone (Figure 6C). Similar effects could be observed for the combination of 20D9-ADC and AC220 in MOLM-13, MV4-11, and MM-6 cells (Figure 6E and supplemental Figure 8C). To calculate synergistic effects, we used 2 calculation methods, the CI42,43 and the ZIP method using the Synergy finder,44,45 which revealed synergism of a combined treatment approach with 20D9-ADC and FLT3-TKI (Figure 6C-F). In the MOLM-13 xenograft model, treatment with midostaurin (50 mg/kg; 5 times weekly × 3) or 20D9-ADC (1 mg/kg; once weekly × 3) as single agents only led to modest growth delay in vivo (Figure 6G). Strikingly, the combination of 20D9-ADC and midostaurin treatment led to drastic tumor reduction and probably cure in 2 out of 3 tumor-bearing animals. Thus, these results indicate a high synergistic potential of the FLT3-specific ADC when combined with FLT3-TKIs.
Discussion
Here, we report the development and preclinical characterization of a novel FLT3-targeting ADC, 20D9-ADC, with robust preclinical activity in multiple models of AML. Furthermore, we found a strong synergistic effect of the combination treatment of 20D9-ADC with a recently approved TKI in FLT3-mutated AML.
FLT3 is an established target for TKIs, like midostaurin, which are approved in FLT3-mutated AMLs with permanently activated receptor signaling.46 Moreover, FLT3 is overexpressed in AML, with a restricted expression pattern in a subset of healthy hematopoietic cells and low abundance in nonhematopoietic tissue. There, a low RNA expression in lung, pancreas, and brain was not yet confirmed to result in cell surface expression of FLT3. Thus, agents targeting FLT3 in AML may have the largest therapeutic index compared with other targets like CD33, CD123, and CLL147 and are expected to have little to no healthy tissue toxicity beyond potential hematologic toxicities.47 This makes FLT3 a promising ADC target in AML treatment, addressing a broader patient cohort regardless of the FLT3 mutation status.
So far, GO, an ADC targeting CD33, is the only approved ADC in AML.48,49 The conjugation and linker design in ADC development are essential as they influence the toxicology profile.8 The linker of GO exhibits instability, leading to premature release of calicheamycin.50 For 20D9-ADC development, the novel P5 conjugation technology with outstanding serum stability was applied to conjugate MMAF via a cleavable linker, which facilitates efficient intracellular release of MMAF.39,51 MMAF belongs, like monomethyl auristatin E, to the microtubule-targeting agents that are used as payloads in two-thirds of all clinical stage ADCs.52 It is a highly potent agent with IC50 in the subnanomolar range and has lower bystander killing effects in comparison with monomethyl auristatin E, which is an advantage in hematologic malignancies.53,54
We show here that 20D9-ADC had strong and selective cytotoxicity in FLT3+ cell lines in vitro. We could clearly distinguish between CD64- and FLT3-mediated cytotoxicity in a cell line model by applying deglycosylated ADCs, showing a strong advantage of co-targeting both receptors. In vivo, we found a dose-dependent response of aggressive AML cell lines to 20D9-ADC independent from the tumor burden at start of treatment, proving the robustness and high efficacy of 20D9-ADC. Interestingly, the deglycosylated 20D9-ADC achieved almost the same efficacy compared with the native 20D9-ADC in MOLM-13 cells in vivo, despite targeting exclusively FLT3. Applying IgG1-ADC in vivo was much less effective than applying 20D9-ADC, indicating that FLT3 targeting might be sufficient and superior compared with dual targeting in the AML mouse model. Moreover, we could also successfully treat AML PDX models in vivo. These PDX samples recapitulate the phenotype of human AML since they comprise AML stem cells and subclonal AML cell populations.32,55 In the in vivo studies, the ADCs were well tolerated as single agent or in combination with TKIs.
To evaluate the toxicity profile of 20D9-ADC in healthy tissue, we focused on hematopoietic stem and progenitor cells, since FLT3 expression in the brain, pancreas, and lung tissue seems to be limited to the cytoplasm or to be very low.47 20D9-ADC in concentrations in the range of IC50 values of AML cell lines did not affect healthy human CD34+ cells, which is promising for a favorable toxicity profile. Only in high concentrations do the 20D9-ADC but also the IgG1-ADC show cytotoxicity toward myelomonocytic and lymphoid progenitors. Thus, Fc receptor engagement might result in side effects and toxicity toward megakaryocytes, leading to thrombocytopenia.48 However, brentuximab vedotin, an approved IgG1-based ADC in Hodgkin lymphoma, showed manageable tolerability and safety profile in a phase 3 study.49 A functional IgG1 Fc region might also have advantages as it was reported that IgG1 can mediate antibody-dependent cell-mediated cytotoxicity and antibody-dependent cellular phagocytosis in the context of drug conjugates.52,56-58 Of note, our studies have shown a superior cytotoxic activity of the native 20D9-ADC compared with the deglycosylated 20D9-ADC (that is devoid of FcgR binding) in vitro but not in vivo. It is unclear whether the FcgR binding properties of the 20D9-ADC will be beneficial in AML patients with respect to toxicity and efficacy. Therefore, further studies in humans or nonhuman primates will be necessary to answer this question. For FLT3 targeting, a favorable toxicity profile can be expected, since a FLT3 x CD3 bispecific antibody in cynomolgues monkey revealed a reversible depletion of dendritic cells, hematopoietic stem and progenitor cells, and monocytes without any major clinical signs of toxicity.47
Due to the observed high efficacy, we evaluated the potential of 20D9-ADC for therapy of FLT3-mutated AML, since especially patients with a high ratio of FLT3-ITD have a worse prognosis.59 By combining 20D9-ADC and FLT3-TKIs, we aimed at (1) exploiting the potential of the FLT3 target, since the ITD-mutated FLT3 receptor has a partially intracellular localization29; (2) opening the therapeutic window for the FLT3-specific ADC treatment while reducing side effects; and (3) integrating an FLT3 ADC in the therapeutic landscape of FLT3-mutated AML.
The combination of 20D9-ADC and TKI treatments showed significantly higher effectivity in vitro compared with single-drug treatment. The in vivo experiments resulted in even more striking benefit of the combination therapy of low-dose 20D9-ADC and midostaurin. We hypothesize that the outstanding treatment efficacy of the drug combination of 20D9-ADC and midostaurin is due to an upregulation of the activated FLT3 receptor on the cell surface as previously reported by our group.29 However, we cannot exclude other mechanisms as midostaurin is not specific for FLT3 and also inhibits other kinases like VEGFR-2, PDGFR, and KIT.60 Furthermore, Li et al described an anti-CD123 ADC to be more efficient in combination with quizartinib,14 and a CD33-targeting ADC (IMGN779) showed increased effectivity in combination with quizartinib.61
Interestingly, an anti-FLT3 ADC from Astellas Pharma (AGS62P1; NCT02864290) is being evaluated in clinical studies, supporting the relevance of the FLT3 receptor as a therapeutic target for ADCs.56 Our study using 20D9-ADC clearly distinguishes from the AGS62P1 molecule. We use ethynylphosphonamidate linkers with excellent stability as opposed to oxime linkages in AGS62P1, which is limited to DAR2, and we believe that the expression profile of FLT3 dictates a higher DAR to ensure a good efficacy also in FLT3 low-expressing malignant cells. Furthermore, the additional CD64 targeting and the combination therapy with TKIs to enhance the efficacy are mostly promising regarding the effectivity in FLT3+ AML.
In conclusion, we have developed and characterized a novel FLT3-targeting ADC that demonstrated potent antileukemic activity in preclinical models of AML, including PDX mouse models. Importantly, 20D9-ADC was effective at low concentrations in combination with midostaurin, suggesting a treatment concept with a possibly favorable toxicity profile. Our data indicate that FLT3 is a clinically promising target for ADC application, which should be further evaluated in clinical studies in combination with FLT3 inhibitors.
Acknowledgments
The authors thank Belay Tizazu for generating Ba/F3 cell models and performing experiments, Maike Fritschle and Annette Frank for animal handling, and Bianka Ksienzyk for cell sorting. The authors acknowledge the iFlow Core Facility of the university hospital Munich (INST 409/225-1 FUGG) for assistance with the generation of flow cytometry data. The authors thank all study participants.
This work was supported by the German Research Foundation (DFG) to H.L., I.J., K.S., and K.S.G. (SFB 1243); to H.L. and A.S. (SPP1623 and GRK1721); and to M.-A.K. and C.P.R.H. (SPP1623) and by German Ministry of Education and Research (BMBF) to K.S., M.A., and H.L. (Project 16GW0360). C.P.R.H. and H.L. were supported by the Leibniz Association within the Leibniz Competition (SAW-2018-FMP-4-P5Label, T18/2017). K.S.G. has received funding from the European Union’s Horizon 2020 Marie Sklodowska-Curie Innovative Training Network (MSCA-ITN, Grant agreement 953407) and German Jose Carreras Leukämiestiftung (DJCLS grant R14). M.S. received research funding from a German Research Foundation (DFG) grant (451580403), SFB-TRR 388/1 2021 -452881907, Bavarian Elite Graduate Training Network, Else-Kröner-Fresenius Stiftung and Bavarian Center for Cancer Research (BZKF). M.R. was member of the IRTG-1243 within the SFB 1243. The authors thank all study participants.
Authorship
Contribution: K.S., H.P., and M.R. conceived the project; M.R. designed and performed experiments, analyzed and interpreted the data, wrote the manuscript, and prepared the figures; E.K. generated the monoclonal antibodies; A.S. chimerized the antibody sequences; H.P., V.W., M.G., S.S., J.S.H., and M.A. performed experiments; N.H., M.F, L.R., and A.L. supported the experiments; T.H. provided expression data; C.P.R.H. and M.-A.K. provided and performed the antibody conjugations; I.J. and B.V. supervised and interpreted mouse experiments and generated transgenic PDX models; H.L., J.H.-S., and D.S. interpreted the data and supported the project plan development; K.S. interpreted the data and coordinated the teams and experiments; C.P.R.H., M.-A.K, I.J., B.V., M.S., and K.S.G. supported by interpreting the data; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: H.L., J.H.-S., D.S., and C.P.R.H. are cofounders of Tubulis GmbH. M.-A.K. S.S., and M.G. are employees at Tubulis GmbH. M.S. received honoraria from Amgen, BMS, Janssen, Kite/Gilead, Roche, Novartis, Pfizer, Celgene, and Takeda. M.S. received research support from Amgen, BMS, Janssen, Kite/Gilead, Miltenyi, MorphoSys, Novartis, Roche, and Seattle Genetics for work unrelated to the manuscript. M.S. declares consultancy for Novartis, Janssen, Amgen, Celgene, Kite/Gilead and Takeda. The presented work is part of a pending patent application by H.L., A.S., M.R., K.S., H.P., J.H.-S., D.S., M.G., C.P.R.H., M.-A.K., I.J., B.V., and E.K. The remaining authors declare no competing financial interests.
The current affiliation for E.K. is Department of Biology II, LMU Munich, Munich, Germany.
Correspondence: Karsten Spiekermann, Medizinische Klinik und Poliklinik III, Marchioninistr 15, 81377 München, Germany; e-mail: karsten.spiekermann@med.uni-muenchen.de.
References
Author notes
For original data, contact the corresponding author, Karsten Spiekermann, at karsten.spiekermann@med.uni-muenchen.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
I.J., H.L., and K.S. contributed equally to this work.