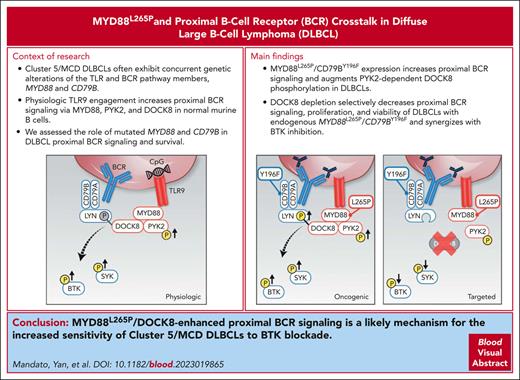
Key Points
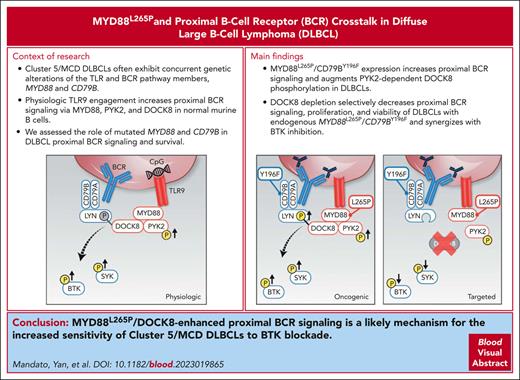
In DLBCLs with MYD88L265P and CD79BY196F alterations, MYD88L265P selectively increased proximal BCR signaling and survival via DOCK8.
MYD88L265P/DOCK8–enhanced proximal BCR signaling is a basis for the increased sensitivity of MYD88L265P/CD79BY196F DLBCLs to BTK blockade.
Abstract
Diffuse large B-cell lymphoma (DLBCL) is a clinically and genetically heterogeneous disease with at least 5 recognized molecular subtypes. Cluster 5 (C5)/MCD tumors frequently exhibit concurrent alterations in the toll-like receptor (TLR) and B-cell receptor (BCR) pathway members, MYD88L265P and CD79B, and have a less favorable prognosis. In healthy B cells, the synergy between TLR and BCR signaling pathways integrates innate and adaptive immune responses and augments downstream NF-κB activation. In addition, physiologic TLR9 pathway engagement via MYD88, protein tyrosine kinase 2 (PYK2), and dedicator of cytokinesis 8 (DOCK8) increases proximal BCR signaling in healthy murine B cells. Although C5/MCD DLBCLs are selectively sensitive to Bruton tyrosine kinase (BTK) inhibition in in vitro studies and certain clinical trials, the role of mutated MYD88 in proximal BCR signaling remains undefined. Using engineered DLBCL cell line models, we found that concurrent MYD88L265P and CD79B alterations significantly increased the magnitude and duration of proximal BCR signaling, at the level of spleen tyrosine kinase and BTK, and augmented PYK2-dependent DOCK8 phosphorylation. MYD88L265P DLBCLs have significantly increased colocalization of DOCK8 with both MYD88 and the proximal BCR-associated Src kinase, LYN, in comparison with MYD88WT DLBCLs, implicating DOCK8 in MYD88L265P/proximal BCR cross talk. Additionally, DOCK8 depletion selectively decreased proximal BCR signaling, cellular proliferation, and viability of DLBCLs with endogenous MYD88L265P/CD79BY196F alterations and increased the efficacy of BTK blockade in these lymphomas. Therefore, MYD88L265P/DOCK8-enhanced proximal BCR signaling is a likely mechanism for the increased sensitivity of C5/MCD DLBCLs to BTK blockade.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoid malignancy in adults and is a clinically and genetically heterogeneous disease.1-4 Among the recognized DLBCL molecular subtypes, cluster 5 (C5)/MCD tumors largely have activated B-cell transcriptional signatures and frequently exhibit concurrent alterations in the toll-like receptor (TLR) and B-cell receptor (BCR) pathway members, MYD88 and CD79B.2-4 In these poor prognosis DLBCLs, MYD88 alterations most commonly involve L265P.2 Concurrent MYD88 and CD79B mutations are also a distinctive feature of primary testicular lymphoma and primary central nervous system lymphoma (PCNSL),5-10 indicating the existence of shared oncogenic mechanisms in these extranodal LBCLs and C5/MCD tumors.
CD79A and CD79B (immunoglobulin α [Igα] and Igβ) proteins associate with BCR and transduce downstream signals through their cytoplasmic immune receptor tyrosine activation motifs (ITAMs).11 After tyrosine phosphorylation by the LYN Src family kinase, the CD79A and CD79B ITAMs provide docking sites for spleen tyrosine kinase (SYK), activating SYK and the downstream BCR signaling cascade.12 Most CD79B mutations perturb the first ITAM tyrosine residue, Y196, and increase cell surface BCR expression.13,14
The MYD88 cytoplasmic adapter is downstream of TLRs (and the interlukin-1 [IL-1] receptor) and is essential for the activation of IL-1 receptor kinases (IRAKs) and NF-κB.15 After TLR engagement, the MYD88 toll/IL-1 receptor (TIR) domain interacts with the TLR cytoplasmic tail.16 A second MYD88 domain forms a multimeric signaling complex with IRAK4/IRAK2, termed the Myddosome.15 The mutation of MYD88 (MYD88L265P) in the TIR domain promotes MYD88 multimerization, increases Myddosome formation, and enhances NF-κB signaling.16-20
In prior murine models, the conditional expression of Myd88L252P (the homolog of human MYD88L265P) led to the development of a low-grade lymphoproliferative disorder and infrequent progression to large cell lymphoma in aged mice.21,22 More recently, murine models of MyD88 and additional C5/MCD alterations, including Cd79b, have been associated with a more rapid onset of a DLBCL-like disorder.23,24 In additional murine in vitro studies, the combination of MYD88L265P and CD79BY196H, but not the individual alterations, promoted B-cell tolerance and autoantibody-secreting plasmablast development.25
Earlier murine models also revealed a basis for synergism between physiologic BCR and TLR9 signaling: fusion of subcellular organelles including TLR9 pathway components and internalized antigen-BCR complexes.26,27 TLR and BCR pathway components are also colocalized to an endolysosomal compartment in human DLBCLs with mutated MYD88.28 Although the identified MYD88, TLR9, and BCR supercomplex, termed “My-T-BCR,” was associated with enhanced downstream NF-κB signaling,28 the potential role of the mutated MYD88 in proximal BCR pathway activation remains undefined.
Jabara et al previously identified the dedicator of cytokinesis 8 (DOCK8) as an adapter that links physiologic TLR signaling with normal BCR activation.29 Earlier DOCK8-deficient murine models revealed a requirement for DOCK8 in germinal center B-cell development, antigen recognition, and B-cell synapse formation.30,31 Loss-of-function mutations in DOCK8 are also associated with a specific type of combined immunodeficiency in humans.32-34
DOCK8 is a member of a family of atypical guanine nucleotide-exchange factors that contains 2 distinct domains, DOCK homology-1 (DHR-1) and DHR-2.29,35,36 The DHR-2 domain binds to and activates guanosine triphosphatases of the Rac-Rho family and modulates CD19 expression, potentially via Wiskott-Aldrich syndrome protein.35-37 DHR-1 directs the protein to the membrane by binding phosphatidyl(3,4,5)triphosphate; this domain also includes a canonical phosphorylation motif for protein tyrosine kinase 2 (PYK2).35,38
In healthy murine B cells, DOCK8 is constitutively complexed with MYD88 and PYK2.29 Upon physiologic TLR9 activation by its CpG DNA ligand, PYK2 phosphorylates DOCK8 putatively in its DHR-1 domain, leading to the recruitment of LYN, activation of SYK, and subsequent downstream phosphorylation of STAT3.29,34 Although DOCK8 mediates the cross talk between physiologic TLR and proximal BCR signaling in normal murine B cells, the role of this adapter in MYD88-mutated B-cell lymphomas is not yet known.
Herein, we identified the contribution of MYD88L265P to proximal BCR signaling in DLBCL and defined a selective dependency on the DOCK8 complex in tumors with concurrent MYD88L265P and CD79B genetic alterations.
Materials and methods
Cell lines
Human DLBCL cell lines OCI-LY1 (LY1) and OCI-LY7 (LY7) were cultured in Iscove Modified Dulbecco medium (Life Technologies, Grand Island, NY), supplemented with 10% heat-inactivated fetal bovine serum (FBS; Sigma, Saint Louis, MO), 2 mM l-glutamine, and 100 U/mL penicillin and 0.1 mg/mL streptomycin (Life Technologies). Human DLBCL cell lines SU-DHL4 (DHL4), HBL1, and TMD8 were cultured in RPMI-1640 medium (Life Technologies) supplemented with 10% FBS, L-glutamine, and penicillin/streptomycin at the aforementioned concentrations. The identities of the DLBCL cell lines were confirmed via the short tandem repeat profiling PowerPlex 1.2 system from Promega (Madison, WI). These cell lines were characterized via whole-exome sequencing, using an expanded bait set as previously described.2
TMA
A tissue microarray (TMA) included triplicate 0.6 mm cores from excisional lymph node biopsies of newly diagnosed, untreated DLBCLs with previously obtained genetic signatures and institutional review board approval (IRB #2010P002736).2,39 Specimens included 4 C5 DLBCLs with MYD88L265P and CD79B mutations and 6 cluster 3 (C3) DLBCLs with wild-type MYD88 (MYD88WT) and CD79B (CD79BWT).2
Antibodies and chemicals
The complete list of the primary antibodies used is presented in supplemental Table 1, available on the Blood website. Secondary anti-mouse and anti-rabbit antibodies conjugated with horseradish peroxidase were obtained from GE HealthCare (Piscataway, NJ). The Bruton tyrosine kinase (BTK) inhibitor ibrutinib (PCI-32765) and the PYK2 inhibitor PF-431396 were purchased from Selleckchem (Houston, TX).
BCR crosslinking
Cells (2.5 × 106 in 250 μL) were crosslinked with AffiniPure F(ab′)2 fragment goat anti-human IgG or IgM (H + L; Jackson Immunoresearch, West Grove, PA) at 6.25 μg/mL IgG (DHL4) and 12 μg/mL IgM (LY1, LY7) for the indicated times at 37°C.
Immunoblotting
Cells were lysed in radioimmunoprecipitation assay buffer containing Halt protease and phosphatase inhibitors (Thermo Fisher Scientific, Rockford, IL) at a ratio of 1 × 106 cells per 100 μL of buffer. Protein concentration was determined using the RC DC (reducing agent and detergent compatible) protein assay (Bio-Rad, Hercules, CA). Samples were separated using a gradient from 4% to 12% NuPage Bis-Tris gels (Invitrogen, Carlsbad, CA) via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes (Thermo Fisher Scientific). Membranes were blocked for 1 hour at room temperature in 5% nonfat dry milk, incubated with primary antibodies at 4°C overnight and secondary antibodies for 1 hour at room temperature. Immunodetection was performed on CL-XPosure films (Thermo Fisher Scientific) using Western Lightning chemiluminescence detection reagents (Perkin Elmer, Waltham, MA).
In-cell western blot
Approximately 2 × 105 cells were seeded in 50 μL serum-free medium in 96-well clear poly-d-lysine plates (Thermo Fisher Scientific) and immobilized at 37°C for 10 minutes before adding 50 μL of 10% FBS medium with or without CpG ODN 2006 (TCgTCgTTTTgTCgTTT-TgTCgTT; Coley Pharmaceutical Group Inc, Wellesley, MA). Cells were fixed with 100 μL per well of 4% paraformaldehyde (PFA) (BioLegend, San Diego, CA), incubated at 37°C for 15 minutes, washed with cold phosphate-buffered saline (PBS) 1×, and permeabilized with intracellular staining permeabilization wash buffer (Perm/Wash; BioLegend, San Diego, CA), followed by blocking with intercept (PBS) blocking buffer (Li-Cor, Lincoln, NE) both for 30 minutes on ice. Primary antibodies were diluted in 50 μL per well of Perm/Wash buffer and incubated for 1 hour to overnight at 4°C. The secondary antibodies were diluted in 50 μL per well of Perm/Wash buffer and incubated for 1 hour at room temperature. The plates were allowed to dry completely before analysis using the Li-Cor Odyssey CLx Imaging System.
Introduction of MYD88L265P and CD79BY196F into DLBCL cell lines
pLVX-MYD88WT-puro and pLVX-MYD88L265P-puro vectors40 were cotransfected with second-generation lentiviral packaging and envelope plasmids pCMV-dR8.91 and pCMV-VSV-G into HEK 293 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After 24 hours and 48 hours, supernatants containing viral particles were collected, and DLBCL cells were infected via spinoculation at 1000 g for 1 hour at 30°C. Infected cells were selected by incubating them for 72 hours with puromycin 1 μg/mL (HBL1), 0.5 μg/mL (TMD8), or 2 μg/mL (DHL4, LY1, and LY7).
The CD79B (c.587A>T) point mutation was generated using CRISPR/Cas9 technology (IDT, Coralville, IA) cotransfecting cells with the following:
crisprRNA 5′-GGAGGAAGATCACACCTACG complexed 1:1 with tracrRNA ATTO 550;
single stranded DNA donor 5′-TCTCCGCCCCCAGGATGACAGCAAGGCTGGCATGGAAGAAG ACCATACATTCGAAGTAAGGAGAGGGGCAGGCCCAGCAGCTCTGAGTCCTCGGGGTCAGT; and
Alt-R Sp Cas9 nuclease, as per manufacturer’s protocol.
ATTO550+ (phycoerythrin+) single cells were sorted into 96 well plates 48 hours after electroporation, and DNA was extracted from each clone after 3 weeks of culture. The polymerase chain reaction products were amplified using the following primers: forward 5′AGGAACACGCTGAAGGATGG and reverse 5′CAAGTTGCTGCCCTGTTGTC. They were sequenced using the primer 5′AGCTCTCGTTGTGGCCTTCT.
Short hairpin RNA–mediated DOCK8 KD
The vectors pLKO.1-puro EmptyT (clone ID: TRCN0000208001) or pLKO.1-puro containing specific DOCK8 short hairpin (sh) RNA sequences (clone IDs: TRCN0000137254 and TRCN0000138603) obtained from Sigma were cotransfected with second-generation lentiviral packaging and envelope plasmids, pCMV-dR8.91 and pCMV-VSV-G, into HEK 293 cells using Lipofectamine 2000. The supernatant, containing viral particles, was collected 48 hours after transduction, and DLBCL cells were infected via spinoculation at 1000 g for 1 hour at 30°C. The infected cells were selected after 72 hours of puromycin treatment 1 μg/mL (HBL1), 0.5 μg/mL (TMD8), or 2 μg/mL (DHL4, LY1, and LY7). Knockdown (KD) efficiency was determined via western blotting at 72 hours after selection.
Proliferation and apoptosis assays
After puromycin selection, the cells were counted, and 100 μL of the cell suspension was added to each well of a round-bottom 96-well plate. Selected samples were treated with ibrutinib 1 nM or 10 nM. Cell growth was assessed using the alamarBlue assay (Invitrogen) for 6 days, as previously described.41 Apoptosis was determined at day 4 (96 hours) via flow cytometric analysis of cells stained with propidium iodide (PI) and annexin V fluorescein isothiocyanate (BD Pharmingen, San Jose, CA) using an LSR Fortessa cytometer (BD Pharmingen). Double-negative cells were considered viable. Annexin V+/PI+ and annexin V+/PI– cell percentages were calculated using FlowJo V10.5.0 (FlowJo LLC, Ashland, OR).
Immunoprecipitation
LY1, LY7, and DHL4 cells (parental; MYD88L265P/CD79BWT and MYD88L265P/CD79BY196F) were treated with a PYK2 inhibitor (PF-431396; 50 nM) for 4 hours before BCR crosslinking for 10 minutes. Cells were harvested and lysed in Triton buffer (1% Triton X-100 with protein inhibitors). Cell lysates were incubated with anti-DOCK8 monoclonal antibody (Santa Cruz) or control IgG overnight at 4°C. Protein A–coupled Sepharose beads were added and incubated for 30 minutes. After multiple washes in Triton buffer, antibody-associated proteins were released by boiling the beads for 5 minutes in LDS sample buffer and resolved via SDS-PAGE. The antibodies listed in supplemental Table 1 were used for immunoblotting.
PLA and confocal microscopy
Lymphoma cells (1 × 104) were seeded on coverslips coated with 0.1% poly-l-lysine (Grace Bio-Labs, Bend, OR) and subsequently maintained in culture media for 20 minutes. Cytoplasmic staining was performed by incubating with BioTracker 555 orange ctyoplasmic membrane dye (Sigma) for 10 minutes at 37°C. Proximity ligation assay (PLA) was performed according to the manufacturer’s instructions, with minor modifications (Olink Bioscience, Uppsala, Sweden). Briefly, cells were washed in PBS 1×, fixed with 4% PFA (Electron Microscopy Sciences, Hatfield, PA) for 15 minutes, permeabilized with 0.2% Triton X-100 in PBS 1× for 10 minutes, incubated with blocking buffer for 1 hour, and subsequently with the primary antibodies overnight. Slides were washed, incubated with secondary antibodies anti-mouse PLUS and anti-rabbit MINUS PLA probes for 1 hour at 37°C, and washed again. Thereafter, the PLUS and MINUS probes were ligated for 30 minutes at 37°C, and the PLA signals were amplified for 100 minutes at 37°C in a humidity chamber. After 3 washes, coverslips were mounted on slides using Vectashield Antifade mounting medium with DAPI (4′,6-diamidino-2-phenylindole) (Vector Labs, Burlingame, CA).
PLA was also performed on formalin-fixed, paraffin-embedded primary DLBCLs in a TMA as previously described.2,39,42 In brief, the slides were incubated at 60°C overnight to melt the paraffin and subsequently transferred to a staining dish for 3 xylene soaks. The slides were then dehydrated with 100% ethanol 3 times and 70% ethanol 3 times and subsequently rehydrated with PBS 3 times. Tissue sections were permeabilized by incubating in 0.5% Triton X-100 in PBS for 10 minutes, followed by incubating in 0.01 M citrate buffer (pH 6.0) at 95°C for 15 minutes to expose protein isotopes. After washing with PBS, the slides were subjected to the aforementioned PLA protocol.
Confocal images were acquired on a Leica SP8 X Confocal Microscope (Leica Microsystems, Deerfield, IL) with a 100× high NA oil objective (Leica Part: HCX PLAPO 100×/1.44 oil CORR CS). PLA signals were detected using Alexa Fluor 488 and cytoplasmic cellular compartments were detected using Alexa Fluor 555. Nuclear boundaries were delineated based on the DAPI staining. All images for a given condition and immunostaining over time were obtained using the same acquisition settings. Mean nuclear intensities were obtained using Leica Application Suite X (LAS X) software. For the PLA in lymphoma cell lines, the number of puncta in 40 to 100 cells was counted in each assay using Cellprofiler software (cellprofiler.org, Broad Institute). PLA signals in the TMA were quantified by measuring the number of puncta present in 3 10 000 μm2 area squares in 3 replicates of each sample. The antibodies used for the PLA assays are listed in supplemental Table 1.
Statistical and graphical analyses
Statistical analyses and graphs were compiled using Graph Pad Prism 9.2.0 (Graph Pad Software, La Jolla, CA). Densitometries were performed with ImageJ (Bethesda, MD).
Results
Physiologic TLR activation augments proximal BCR signaling in DLBCL
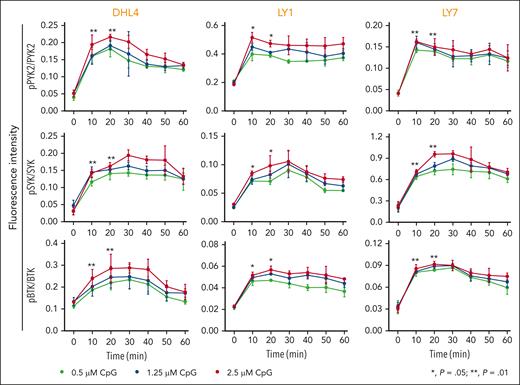
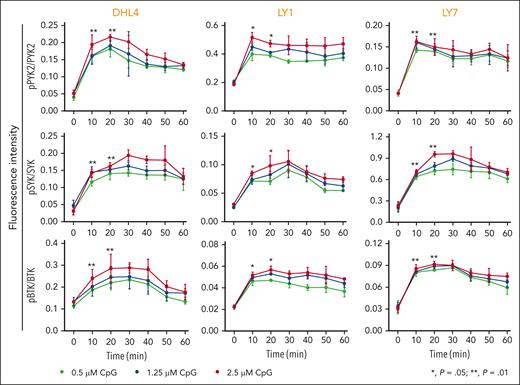
We first assessed the consequences of physiologic TLR9 activation in 3 DLBCL cell lines with intact BCR signaling43 and no genetic alterations in MYD88,44 DHL4, LY1, or LY7. The cell lines were treated with CpG DNA (0.5 μM, 1.25 μM, and 2.5 μM) and subsequently evaluated at serial time points for PYK2, SYK, and BTK phosphorylation. In all 3 lines, CpG treatment induced the phosphorylation of PYK2 (Y402), SYK (Y525/526), and BTK (Y223) (Figure 1), highlighting the cross talk between physiologic TLR activation and proximal BCR signaling in DLBCLs with MYD88WT.
Physiologic TLR activation augments proximal BCR signaling in DLBCL. Line graphs of the normalized fluorescence intensity of pPYK2/PYK2, pSYK/SYK, and pBTK/BTK in DHL4, LY1, and LY7 cells treated with CpG (0.5, 1.25, and 2.5 μM) for 60 minutes and analyzed using in-cell western blot. Error bars represent the standard deviation of 3 independent replicates from a representative experiment. The statistical significance of the changes in fluorescence intensity at 10 and 20 minutes compared with that at baseline (time 0) was measured using the Mann-Whitney test. ∗P = .05; ∗∗P = .01.
Physiologic TLR activation augments proximal BCR signaling in DLBCL. Line graphs of the normalized fluorescence intensity of pPYK2/PYK2, pSYK/SYK, and pBTK/BTK in DHL4, LY1, and LY7 cells treated with CpG (0.5, 1.25, and 2.5 μM) for 60 minutes and analyzed using in-cell western blot. Error bars represent the standard deviation of 3 independent replicates from a representative experiment. The statistical significance of the changes in fluorescence intensity at 10 and 20 minutes compared with that at baseline (time 0) was measured using the Mann-Whitney test. ∗P = .05; ∗∗P = .01.
MYD88L265P and CD79BY196F coexpression augments proximal BCR signaling in DLBCL
Given the concurrent MYD88L265P/CD79B mutations in a subset of primary DLBCLs (supplemental Figure 1A), we postulated that MYD88L265P might similarly augment proximal BCR signaling in DLBCLs in the absence of physiologic (CpG-induced) TLR9 activation. Therefore, we introduced MYD88WT or MYD88L265P into the DHL4, LY1, and LY7 cell lines through viral transduction (supplemental Figure 2A). The engineered MYD88L265P DLBCL cell lines expressed lower levels of the NF-κB inhibitor IκBα and increased abundance of the NF-κB targets BFL1 and CD40 (supplemental Figure 2B-C), providing functional evidence of TLR and NF-κB pathway activation.
To explore the significance of concurrent MYD88 and CD79B alterations, we used CRISPR/Cas9 to edit CD79B (c.587A>T) in the MYD88WT and MYD88L265P DHL4, LY1, and LY7 cell lines (supplemental Figure 3). The resulting engineered DLBCL cell lines expressed MYD88WT/CD79BY196F or MYD88L265P/CD79BY196F.
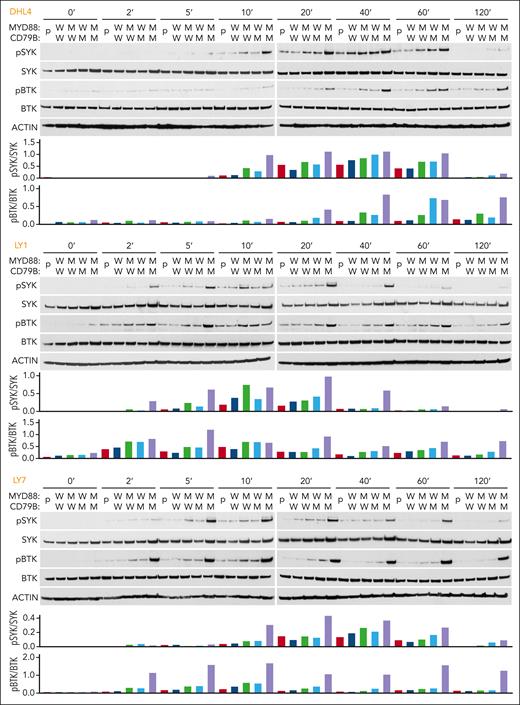
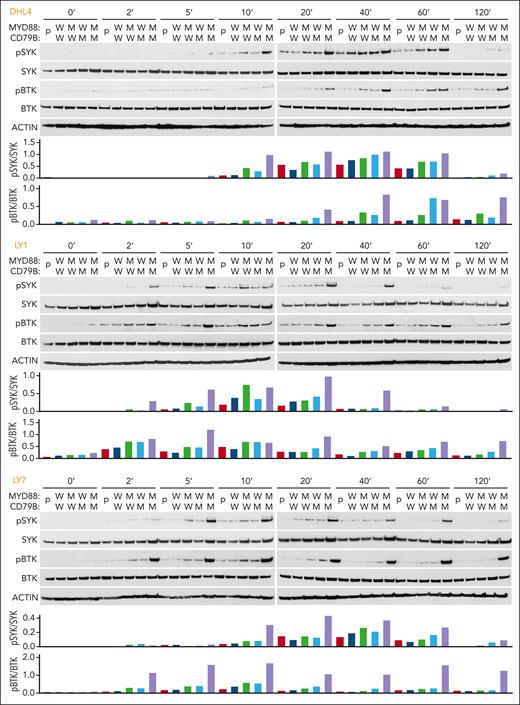
Next, we BCR-crosslinked the engineered DLBCL cell lines with anti-IgG (DHL4) or anti-IgM (LY1 and LY7) F(ab′)2 fragments. In all 3 cell lines, concurrent mutations of MYD88L265P and CD79BY196F increased the magnitude and duration of SYK (Y525/526) and BTK (Y223) phosphorylation (Figure 2). These results suggest that MYD88L265P augments proximal BCR signaling in the absence of physiologic TLR9 activation and concurrent MYD88L265P and CD79BY196F alterations further increase BCR activation (Figure 2).
MYD88L265P and CD79BY196F coexpression augments proximal BCR signaling in DLBCL. For each cell line: (Top) Immunoblot of pSYK, SYK, pBTK, and BTK in engineered DHL4, LY1, and LY7 cells at baseline and 2, 5, 10, 20, 40, 60, and 120 minutes after BCR crosslinking. (Bottom) Densitometry analysis of protein bands. Actin was used as a loading control. Data are representative of 1 of 3 independent experiments.
MYD88L265P and CD79BY196F coexpression augments proximal BCR signaling in DLBCL. For each cell line: (Top) Immunoblot of pSYK, SYK, pBTK, and BTK in engineered DHL4, LY1, and LY7 cells at baseline and 2, 5, 10, 20, 40, 60, and 120 minutes after BCR crosslinking. (Bottom) Densitometry analysis of protein bands. Actin was used as a loading control. Data are representative of 1 of 3 independent experiments.
MYD88L265P expression increases PYK2-associated DOCK8 phosphorylation
In normal B cells, physiologic TLR signaling augments phosphorylation of PYK2-associated DOCK8, recruitment of the Src kinase LYN, and subsequent activation of SYK.29 Therefore, we investigated whether MYD88L265P similarly modulated PYK2 activity and DOCK8 phosphorylation in the engineered DLBCL cell lines.
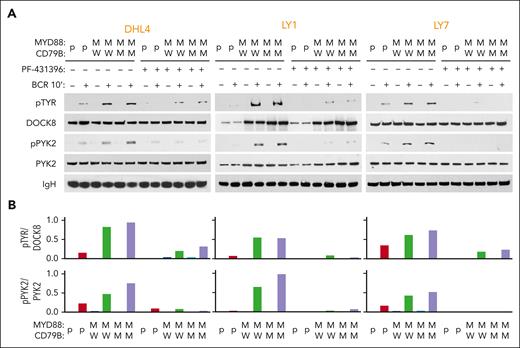
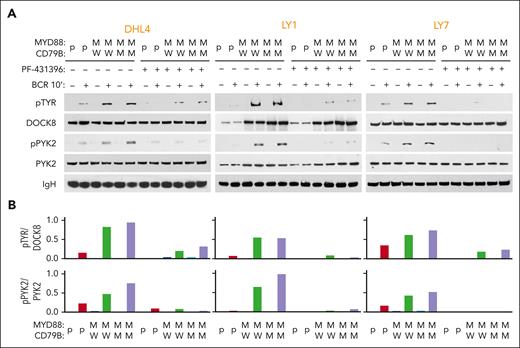
We examined phospho-DOCK8 levels in parental and engineered MYD88L265P/CD79BWT and MYD88L265P/CD79BY196F DHL4, LY1, and LY7 cell lines at baseline and after BCR crosslinking (Figure 3). As there were no available phospho-DOCK8 antibodies, we immunoprecipitated DOCK8 and immunoblotted the size-fractionated proteins with anti–phospho-tyrosine (Figure 3A, top). DOCK8 phosphorylation was markedly higher in engineered MYD88L265P cell lines (with CD79BY196F or CD79BWT) than in parental cells (Figure 3A-B, top). PYK2 (Y402) autophosphorylation levels were also increased in DOCK8 immunoprecipitates from engineered MYD88L265P cell lines (Figure 3A-B, bottom). Pretreatment with the dual focal adhesion kinase/PYK2 inhibitor PF-431396 strikingly reduced both PYK2 and DOCK8 phosphorylation in DLBCLs (Figure 3A-B), confirming the specificity of the observed effects. These findings associate DOCK8 and PYK2 in the signaling cascade and potentially implicate DOCK8 in the cross talk between MYD88L265P and proximal BCR signaling in DLBCL. In primary DLBCLs, neither DOCK8 nor PYK2 was recurrently mutated (supplemental Figure 1B-C), suggesting that these downstream signaling pathway members are mainly modulated by interactions with MYD88L265P.
MYD88L265P expression increases PYK2-dependent DOCK8 phosphorylation in DLBCL. (A) Immunoblot of pTYR, DOCK8, pPYK2, and PYK2 in engineered DHL4, LY1, and LY7 cells that were untreated or treated with 50 nM PYK2 inhibitor (PF-431396) before analysis at baseline or after BCR crosslinking for 10 minutes. IgH was used as a loading control. Data are representative of 1 of 3 independent experiments. (B) Densitometry analysis of the protein bands.
MYD88L265P expression increases PYK2-dependent DOCK8 phosphorylation in DLBCL. (A) Immunoblot of pTYR, DOCK8, pPYK2, and PYK2 in engineered DHL4, LY1, and LY7 cells that were untreated or treated with 50 nM PYK2 inhibitor (PF-431396) before analysis at baseline or after BCR crosslinking for 10 minutes. IgH was used as a loading control. Data are representative of 1 of 3 independent experiments. (B) Densitometry analysis of the protein bands.
MYD88L265P increases the colocalization of MYD88 with DOCK8 and DOCK8 with LYN
In normal B cells, physiologic TLR signaling increases the association between DOCK8, MYD88, and PYK2.29 We postulated that MYD88L265P might similarly stabilize the DOCK8 complex and used PLAs, which detect protein-protein interactions at less than 40 nm in situ, to assess the colocalization of MYD88 and DOCK8 in parental (p) and engineered MYD88WT (W) and MYD88L265P (M) DLBCL cell lines. There were significantly increased DOCK8:MYD88 PLA signals in MYD88L265P DHL4, LY1, and LY7 cell lines as compared with those in MYD88WT or parental cells (Figure 4A).
MYD88L265P expression increases the colocalization of MYD88 with DOCK8 and DOCK8 with LYN in DLBCL. (A) (Top) Confocal images of MYD88:DOCK8 PLA signals in DHL4, LY1, and LY7 parental (p), MYD88WT (W)-, or MYD88L265P (M)-overexpressing cells. (Bottom) Quantification of PLA signals in n > 60 cells classified per condition. Violin plots, ∗∗P < .01; ∗∗∗∗P < .0001. (B) (Top) Confocal images of LYN:DOCK8 PLA signals in DHL4, LY1, and LY7 p, W-, or M-overexpressing cells. (Bottom) Quantification data of n > 40 cells classified per condition. Violin plots, ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001. Asterisks represent P values in a Mann-Whitney test.
MYD88L265P expression increases the colocalization of MYD88 with DOCK8 and DOCK8 with LYN in DLBCL. (A) (Top) Confocal images of MYD88:DOCK8 PLA signals in DHL4, LY1, and LY7 parental (p), MYD88WT (W)-, or MYD88L265P (M)-overexpressing cells. (Bottom) Quantification of PLA signals in n > 60 cells classified per condition. Violin plots, ∗∗P < .01; ∗∗∗∗P < .0001. (B) (Top) Confocal images of LYN:DOCK8 PLA signals in DHL4, LY1, and LY7 p, W-, or M-overexpressing cells. (Bottom) Quantification data of n > 40 cells classified per condition. Violin plots, ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001. Asterisks represent P values in a Mann-Whitney test.
As phosphorylated DOCK8 recruits LYN,29 we next assessed the colocalization of these 2 proteins in the same parental (p), MYD88WT (W), and MYD88L265P (M) DLBCL cell lines. We detected significantly increased DOCK8:LYN PLA signals in DLBCL cells that expressed MYD88L265P, rather than MYD88WT or the endogenous protein (p) (Figure 4B). These findings provide direct evidence of an enhanced association between MYD88L265P and DOCK8 and an increased interaction between DOCK8 and LYN in MYD88L265P DLBCLs.
MYD88L265P expression increases endolysosomal colocalization of MYD88 and DOCK8
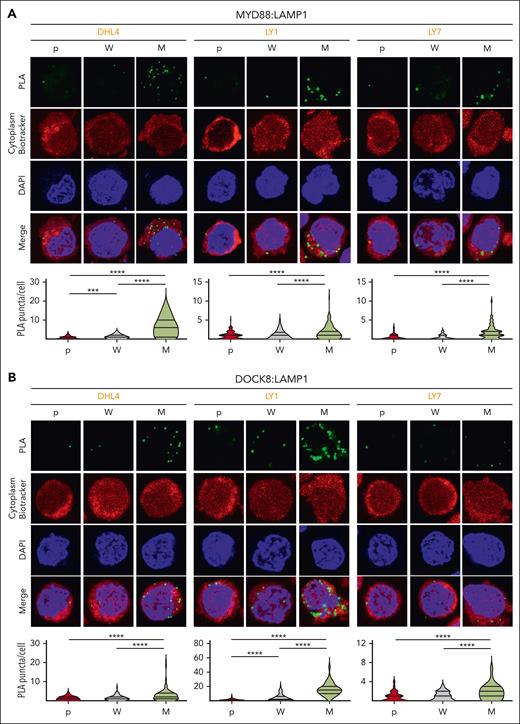
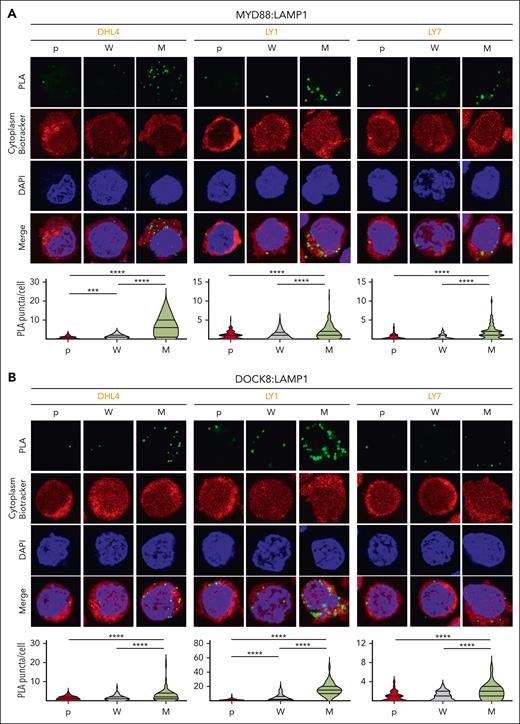
Phelan et al previously described a multiprotein complex including TLR and BCR signaling pathway components that was significantly more abundant in DLBCLs with MYD88L265P than in those with MYD88WT.28 The My-T-BCR complex, first identified because of its colocalization with LAMP1+ vesicles, was thought to reflect BCR shuttling from the plasma membrane to the endolysosomal site of complex formation.28 Therefore, we evaluated the colocalization of MYD88 and DOCK8 with LAMP1 in parental, MYD88WT (W) and MYD88L265P (M) DLBCL cell lines with PLAs. There were significantly increased MYD88:LAMP1 (Figure 5A) and DOCK8:LAMP1 (Figure 5B) PLA signals in MYD88L265P DLBCL cell lines compared with those in MYD88WT or parental cells, localizing MYD88L265P:DOCK8 interactions to the proximity of the endolysosomes.
MYD88L265Pexpression increases endolysosomal colocalization of MYD88 and DOCK8. (A) (Top) Confocal images of MYD88:LAMP1 PLA signals in DHL4, LY1, and LY7 parental (p), MYD88WT (W)-, or MYD88L265P (M)-overexpressing cells. (Bottom) Quantification data of n > 50 cells classified per condition. Violin plots, ∗∗∗P < .001; ∗∗∗∗P < .0001. (B) (Top) Confocal images of LAMP1:DOCK8 PLA signals in DHL4, LY1, and LY7 p, W-, or M-overexpressing cells. (Bottom) Quantification data of n > 70 cells classified per condition. Violin plots, ∗∗∗∗P < .0001. Asterisks represent P values in a Mann-Whitney test.
MYD88L265Pexpression increases endolysosomal colocalization of MYD88 and DOCK8. (A) (Top) Confocal images of MYD88:LAMP1 PLA signals in DHL4, LY1, and LY7 parental (p), MYD88WT (W)-, or MYD88L265P (M)-overexpressing cells. (Bottom) Quantification data of n > 50 cells classified per condition. Violin plots, ∗∗∗P < .001; ∗∗∗∗P < .0001. (B) (Top) Confocal images of LAMP1:DOCK8 PLA signals in DHL4, LY1, and LY7 p, W-, or M-overexpressing cells. (Bottom) Quantification data of n > 70 cells classified per condition. Violin plots, ∗∗∗∗P < .0001. Asterisks represent P values in a Mann-Whitney test.
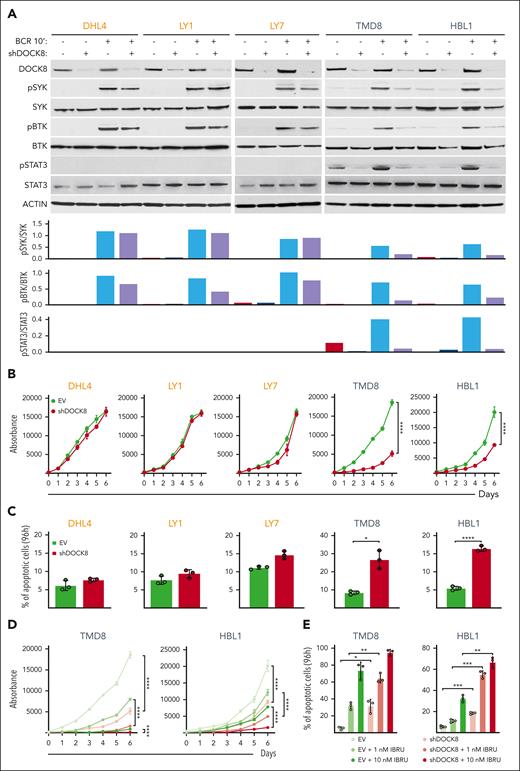
DOCK8 depletion selectively decreases proximal BCR signaling, proliferation, and viability of DLBCLs with endogenous MYD88L265P/CD79BY196F alterations
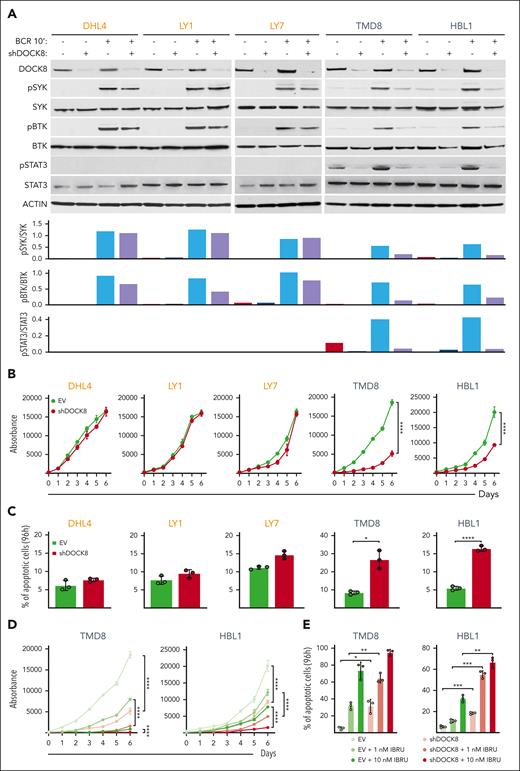
After implicating DOCK8 in enhanced proximal BCR signaling in the engineered DLBCL cell lines (Figures 3-5), we assessed the consequences of DOCK8 depletion in DLBCL cell lines with endogenous MYD88L265P and CD79BY196F alterations (TMD8 and HBL1) or endogenous MYD88WT and CD79BWT (DHL4, LY1, and LY7). The 5 cell lines were transduced with the empty vector or a combination of 2 shDOCK8–expressing vectors, BCR crosslinked, and subsequently analyzed for SYK and BTK phosphorylation. DOCK8 KD selectively decreased SYK and BTK phosphorylation in DLBCL cell lines with endogenous MYD88L265P/CD79BY196F (TMD8 and HBL1) but had little to no effect in lines with endogenous MYD88WT and CD79BWT (DHL4, LY1, and LY7; Figure 6A). As expected, we did not detect STAT3 phosphorylation at baseline or after BCR crosslinking in cell lines with endogenous MYD88WT and CD79BWT (DHL4, LY1, and LY7; Figure 6A). In contrast, BCR crosslinking augmented STAT3 phosphorylation in cell lines with endogenous MYD88L265P/CD79BY196F mutations (TMD8 and HBL1), and DOCK8 depletion markedly reduced STAT3 activation (Figure 6A). Similar results for SYK, BTK, and STAT3 phosphorylation were obtained in a genetically engineered DLBCL cell line with introduced MYD88L265P and CD79BY196F mutations (supplemental Figure 4). These findings define a selective role for DOCK8 in MYD88L265P/CD79BY196F-enhanced proximal BCR signaling and downstream activation of STAT3. Additionally, DOCK8 depletion selectively inhibited proliferation and increased apoptosis of MYD88L265P/CD79BY196F DLBCL cell lines (TMD8 and HBL1) but not DLBCLs with endogenous MYD88WT and CD79BWT (DHL4, LY1, and LY7; Figure 6B-C).
DOCK8 depletion selectively decreases proximal BCR signaling, cellular proliferation, and viability in DLBCLs with endogenous MYD88L265P/CD79BY196F alterations and synergizes with chemical BTK inhibition. (A) (Top) Immunoblot of DOCK8 in parental DHL4, LY1, and LY7 cell lines with endogenous MYD88WT (and CD79BWT), and TMD8 and HBL1 cell lines with endogenous MYD88L265P and CD79BY196F after shRNA–mediated DOCK8 depletion. Phosphorylation of SYK, BTK, and STAT3 was analyzed at baseline and after BCR crosslinking for 10 minutes. Actin was used as a loading control. (Bottom) Densitometry analysis of protein bands. Data are representative of 1 of 3 independent experiments. (B) Proliferation of the indicated DLBCL cell lines, transduced with an empty vector (EV) or short hairpin DOCK8 (shDOCK8), was detected using the alamarBlue assay. P values for shDOCK8 vs EV were determined with a 2-way analysis of variance (ANOVA); ∗∗∗∗P < .0001. Error bars represent the standard deviation (SD) of 3 independent replicates from a representative experiment. (C) Apoptosis of the indicated cell lines at day 4 (96 hours) is shown as percentage of annexin V+ cells ± SD from 3 independent replicates of a representative experiment; ∗P < .05; ∗∗∗∗P < .0001. Asterisks represent P values in the Student t test. (D) Proliferation of TMD8 and HBL1 cells transduced with the EV control or shDOCK8 and treated with the indicated doses of IBRU for 6 days, as detected via the alamarBlue assay. P values were determined using 2-way ANOVA; ∗∗∗∗P < .0001. Error bars represent the SD of 3 independent replicates from a representative experiment. (E) Apoptosis of TMD8 and HBL1 cells (in D) on day 4 (96 hours) is shown as the percentage of annexin V+ cells ± SD of 3 independent replicates from a representative experiment: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Asterisks represent P values in the Student t test. IBRU, ibrutinib.
DOCK8 depletion selectively decreases proximal BCR signaling, cellular proliferation, and viability in DLBCLs with endogenous MYD88L265P/CD79BY196F alterations and synergizes with chemical BTK inhibition. (A) (Top) Immunoblot of DOCK8 in parental DHL4, LY1, and LY7 cell lines with endogenous MYD88WT (and CD79BWT), and TMD8 and HBL1 cell lines with endogenous MYD88L265P and CD79BY196F after shRNA–mediated DOCK8 depletion. Phosphorylation of SYK, BTK, and STAT3 was analyzed at baseline and after BCR crosslinking for 10 minutes. Actin was used as a loading control. (Bottom) Densitometry analysis of protein bands. Data are representative of 1 of 3 independent experiments. (B) Proliferation of the indicated DLBCL cell lines, transduced with an empty vector (EV) or short hairpin DOCK8 (shDOCK8), was detected using the alamarBlue assay. P values for shDOCK8 vs EV were determined with a 2-way analysis of variance (ANOVA); ∗∗∗∗P < .0001. Error bars represent the standard deviation (SD) of 3 independent replicates from a representative experiment. (C) Apoptosis of the indicated cell lines at day 4 (96 hours) is shown as percentage of annexin V+ cells ± SD from 3 independent replicates of a representative experiment; ∗P < .05; ∗∗∗∗P < .0001. Asterisks represent P values in the Student t test. (D) Proliferation of TMD8 and HBL1 cells transduced with the EV control or shDOCK8 and treated with the indicated doses of IBRU for 6 days, as detected via the alamarBlue assay. P values were determined using 2-way ANOVA; ∗∗∗∗P < .0001. Error bars represent the SD of 3 independent replicates from a representative experiment. (E) Apoptosis of TMD8 and HBL1 cells (in D) on day 4 (96 hours) is shown as the percentage of annexin V+ cells ± SD of 3 independent replicates from a representative experiment: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Asterisks represent P values in the Student t test. IBRU, ibrutinib.
In contrast to the selective effects of DOCK8 depletion on SYK and BTK phosphorylation, cell proliferation, and apoptosis in DLBCL cell lines with endogenous MYD88L265P/CD79BY196F alterations (Figure 6A-C), DOCK8 KD similarly decreased CD19 abundance in all analyzed lines (supplemental Figure 5). Therefore, DOCK8 DHR-2 domain–associated modulation of CD19 expression does not explain the selective effects of DOCK8 depletion in MYD88L265P/CD79BY196F DLBCLs. Instead, PYK2 modulation of the previously described DOCK8 DHR-1 motif29,38 more closely aligns with augmented proximal BCR signaling in MYD88L265P/CD79BY196F DLBCLs.
DOCK8 depletion augments the efficacy of chemical BTK inhibition in MYD88L265P/CD79BY196F DLBCLs
We and others previously found that DLBCL cell lines with endogenous MYD88L265P/CD79BY196F alterations, HBL1 and TMD8, were particularly sensitive to chemical BTK inhibition with ibrutinib at low nanomolar doses.13,40,44 Given the selective antiproliferative and cytotoxic effects of DOCK8 KD in MYD88L265P/CD79BY196F DLBCLs (Figure 6B-C), we also assessed the combined chemical BTK inhibition and DOCK8 depletion in these cell lines. Chemical BTK inhibition more effectively decreased BCR crosslink–induced BTK and STAT3 phosphorylation in DOCK8-depleted cells than in the parental cells (supplemental Figure 6). The antiproliferative and cytotoxic effects of chemical BTK inhibition (ibrutinib, 1 nM and 10 nM) were also significantly greater in DOCK8-depleted TMD8 and HBL1 cells than in control cells (Figure 6D-E). These data highlight the potential benefit of targeting DOCK8-dependent MYD88L265P-associated proximal BCR signaling in DLBCLs with concurrent MYD88L265P/CD79BY196F alterations.
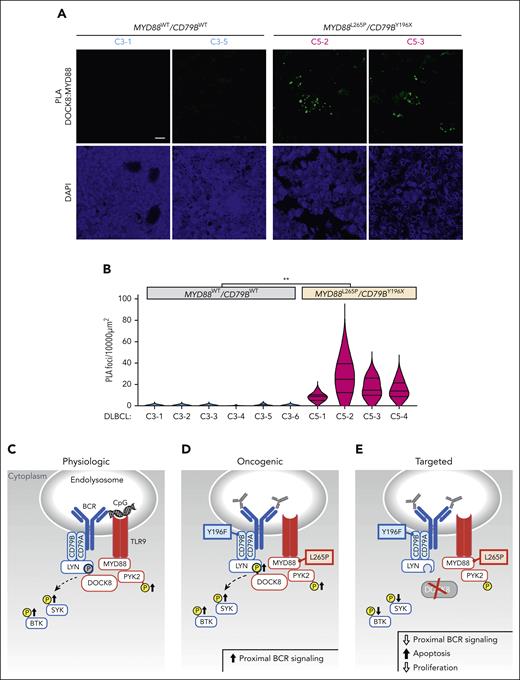
Colocalization of MYD88L265P with DOCK8 in primary DLBCLs
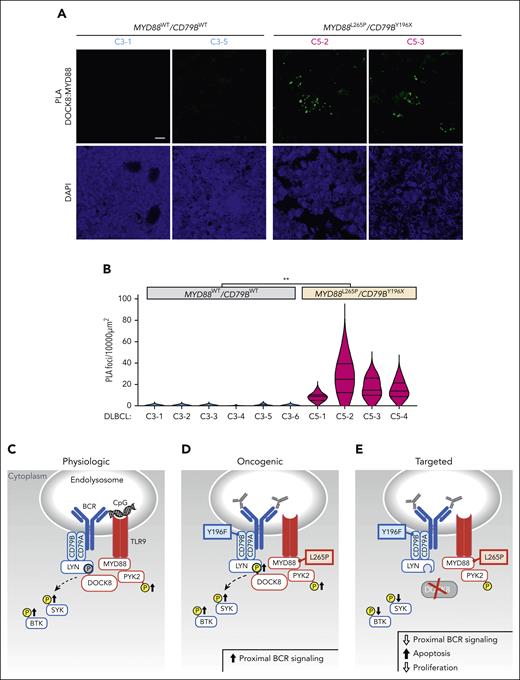
After characterizing the coassociation between MYD88L265 and DOCK8 and the functional consequences of DOCK8 depletion in DLBCL cell lines, we extended the analyses to genetically defined primary tumors. We performed PLA analyses of the coassociation between MYD88L265P (or MYD88WT) and DOCK8 in 10 newly diagnosed, previously untreated primary DLBCLs, including 6 C3 tumors with MYD88WT/CD79BWT and 4 C5 DLBCLs with MYD88L265P/CD79BY196X alterations. MYD88:DOCK8 PLA signals were significantly more abundant in C5 MYD88L265P/CD79BY196X DLBCLs than in C3 MYD88WT/CD79BWT tumors (Figure 7A-B), extending our findings to primary DLBCLs.
Colocalization of MYD88 and DOCK8 in primary DLBCLs and schema of augmented proximal BCR signaling in MYD88L265P/CD79BY196X DLBCLs. (A) Confocal images of MYD88:DOCK8 PLA signals in 2 representative C3 (MYD88WT/CD79BWT) and 2 representative C5 (MYD88L265P/CD79BY196X) primary DLBCLs. Scale bar, 10 μm. (B) Quantification of PLA signals in primary DLBCLs. PLA puncta in 10 000 μm2 areas from triplicate core biopsies from 6 C3 (MYD88WT/CD79BWT) and 4 C5 (MYD88L265P/CD79BY196X) primary DLBCLs were scored. Violin plots, ∗∗P < .01. Asterisks represent P values in a Mann-Whitney test. (C) Physiologic signaling. CpG-induced TLR signaling augments PYK2 phosphorylation and phosphorylation of the proximal BCR kinases SYK and BTK. DOCK8 phosphorylation, shown in gray, was previously demonstrated.29 (D) Oncogenic signaling. MYD88L265P increases PYK2 and DOCK8 phosphorylation and phosphorylation of SYK and BTK. (E) Therapeutic targeting. DOCK8 depletion decreases SYK and BTK phosphorylation and is selectively antiproliferative and cytotoxic in MYD88L265P/CD79BY196F cell lines. TLR pathway members are shown in red; BCR pathway members are shown in blue.
Colocalization of MYD88 and DOCK8 in primary DLBCLs and schema of augmented proximal BCR signaling in MYD88L265P/CD79BY196X DLBCLs. (A) Confocal images of MYD88:DOCK8 PLA signals in 2 representative C3 (MYD88WT/CD79BWT) and 2 representative C5 (MYD88L265P/CD79BY196X) primary DLBCLs. Scale bar, 10 μm. (B) Quantification of PLA signals in primary DLBCLs. PLA puncta in 10 000 μm2 areas from triplicate core biopsies from 6 C3 (MYD88WT/CD79BWT) and 4 C5 (MYD88L265P/CD79BY196X) primary DLBCLs were scored. Violin plots, ∗∗P < .01. Asterisks represent P values in a Mann-Whitney test. (C) Physiologic signaling. CpG-induced TLR signaling augments PYK2 phosphorylation and phosphorylation of the proximal BCR kinases SYK and BTK. DOCK8 phosphorylation, shown in gray, was previously demonstrated.29 (D) Oncogenic signaling. MYD88L265P increases PYK2 and DOCK8 phosphorylation and phosphorylation of SYK and BTK. (E) Therapeutic targeting. DOCK8 depletion decreases SYK and BTK phosphorylation and is selectively antiproliferative and cytotoxic in MYD88L265P/CD79BY196F cell lines. TLR pathway members are shown in red; BCR pathway members are shown in blue.
Discussion
Herein, we explored the potential molecular advantages of concurrent MYD88L265P and CD79B alterations in DLBCL. In the engineered DLBCL cell line models, concurrent MYD88L265P and CD79B alterations significantly increased the magnitude and duration of proximal BCR signaling as well as PYK2-dependent DOCK8 phosphorylation (Figure 7C-E). In comparison to MYD88WT DLBCLs, MYD88L265P DLBCLs had significantly increased colocalization of DOCK8 with both MYD88 and the BCR-associated Src kinase LYN and increased colocalization of MYD88 and DOCK8 in the proximity of endolysosomes. These findings implicate DOCK8 in the cross talk between MYD88L265P and proximal BCR kinases and localize DOCK8 with intracellular TLR9/BCR signaling components in MYD88L265P DLBCLs. Most importantly, DOCK8 depletion selectively decreased proximal BCR signaling, cellular proliferation, and viability of DLBCLs with endogenous MYD88L265P/CD79BY196F alterations (Figure 7C-E) and increased the efficacy of chemical BTK inhibition in these lymphomas. Together, these studies define a selective role for MYD88L265P, via DOCK8, in proximal BCR signaling and survival of DLBCLs with concurrent MYD88L265P/CD79B alterations.
The synergy between the TLR and BCR signaling pathways in normal B cells integrates innate and adaptive immune responses and augments downstream NF-κB activation.45-47 Jabara et al29 defined an additional role for physiological TLR9 pathway engagement, via MYD88, PYK2, and DOCK8, in proximal BCR signaling at the level of SYK. Others have recently described enhanced association and activation of SYK in MYD88-mutated lymphoma cells.48 Our findings extend these observations to oncogenic MYD88L265P- and DOCK8-augmented proximal BCR signaling in DLBCLs with concurrent MYD88L265P/CD79B alterations. As these tumors respond less favorably to standard R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) induction regimens,2,4,49 our data have important implications for targeted therapy.
MYD88L265P/DOCK8-enhanced proximal BCR signaling, at the level of SYK and BTK, provides an additional mechanistic basis for the increased sensitivity of MCD DLBCLs to BTK blockade.50 In younger patients who were less likely to experience treatment-related toxicity and had MCD DLBCLs, adding ibrutinib to R-CHOP induction therapy increased event-free survival.50 Additionally, the success of BTK inhibition in clinical trials51 of patients with PCNSLs characterized by MYD88L265P/CD79B alterations5,6,9,10 may be associated with MYD88L265P/DOCK8–enhanced proximal BCR signaling.
The fact that MYD88L265P augments proximal BCR signaling, in addition to TLR pathway activity, also influences the selection of treatment targets. For example, inhibition of downstream TLR pathway components, such as IRAK4,52 is unlikely to block MYD88L265P/DOCK8-enhanced proximal BCR signaling. This may explain the limited efficacy of IRAK4 kinase inhibitors and IRAK4 selective degraders in transcriptionally defined activated B-cell DLBCLs.52
DOCK8-dependent, MYD88L265P-associated proximal BCR signaling may be another selective vulnerability in DLBCLs with concurrent MYD88L265P/CD79B alterations, such as C5/MCD tumors.2,4 DOCK8 depletion selectively decreased proximal BCR signaling and cellular viability in MYD88L265P/CD79BY196X DLBCLs but not in DLBCLs with wild-type MYD88 and CD79B. Furthermore, DOCK8 depletion significantly increased the efficacy of BTK blockade in these MYD88L265P/CD79BY196X DLBCLs. However, given the role of DOCK8 in additional immune cells,53-57 the optimal approach to targeting DOCK8-dependent, MYD88L265P-associated proximal BCR signaling requires careful consideration.
Taken together, our studies reveal the role of MYD88L265P- and DOCK8-augmented proximal BCR signaling in genetically defined, poor prognosis DLBCLs and inform current and future treatment strategies.
Acknowledgments
This work was supported by a Lymphoma Research Foundation Postdoctoral Fellowship grant (816777) (E.M.) and a Leukemia & Lymphoma Society Translational Research Program grant (6613-20) (M.A.S.).
Authorship
Contribution: E.M., Q.Y., Y.H., and M.A.S. designed the study; E.M., Q.Y., J.O., J.P., Y.Q., J.H., K.B., and Y.H. performed the experiments; E.M., Q.Y., J.O., J.P., Y.Q., B.C., S.J.R., S.J.K., R.A.R., and M.A.S. analyzed the data; E.M., Q.Y., S.J.K., and M.A.S. wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: M.A.S. received research funding from Bayer, Bristol Myers Squibb, AstraZeneca, and AbbVie and served as a scientific adviser to AstraZeneca. S.J.R. has received research support from Affimed, Merck, and Bristol Myers Squibb, and is a member of the Scientific Advisory Board for Immunitas Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Margaret A. Shipp, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Mayer 513, Boston, MA 02215; e-mail: margaret_shipp@dfci.harvard.edu.
References
Author notes
∗E.M. and Q.Y. contributed equally to this work.
Data are available on request from the corresponding author, Margaret A. Shipp (margaret_shipp@dfci.harvard.edu).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.