Key Points
GPVI is a key player in LPS-induced pulmonary thrombo-inflammation by promoting PNC formation, neutrophil recruitment, and NETosis.
Anti-GPVI treatment protects mice from LPS-induced ALI and respiratory failure.
Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are associated with high morbidity and mortality. Excessive neutrophil infiltration into the pulmonary airspace is the main cause for the acute inflammation and lung injury. Platelets have been implicated in the pathogenesis of ALI/ARDS, but the underlying mechanisms are not fully understood. Here, we show that the immunoreceptor tyrosine-based activation motif–coupled immunoglobulin-like platelet receptor, glycoprotein VI (GPVI), plays a key role in the early phase of pulmonary thrombo-inflammation in a model of lipopolysaccharide (LPS)-induced ALI in mice. In wild-type (WT) control mice, intranasal LPS application triggered severe pulmonary and blood neutrophilia, hypothermia, and increased blood lactate levels. In contrast, GPVI-deficient mice as well as anti-GPVI–treated WT mice were markedly protected from pulmonary and systemic compromises and showed no increased pulmonary bleeding. High-resolution multicolor microscopy of lung sections and intravital confocal microcopy of the ventilated lung revealed that anti-GPVI treatment resulted in less stable platelet interactions with neutrophils and overall reduced platelet–neutrophil complex (PNC) formation. Anti-GPVI treatment also reduced neutrophil crawling and adhesion on endothelial cells, resulting in reduced neutrophil transmigration and alveolar infiltrates. Remarkably, neutrophil activation was also diminished in anti-GPVI–treated animals, associated with strongly reduced formation of PNC clusters and neutrophil extracellular traps (NETs) compared with that in control mice. These results establish GPVI as a key mediator of neutrophil recruitment, PNC formation, and NET formation (ie, NETosis) in experimental ALI. Thus, GPVI inhibition might be a promising strategy to reduce the acute pulmonary inflammation that causes ALI/ARDS.
Introduction
Acute lung injury (ALI) and, its more severe form, acute respiratory distress syndrome (ARDS) are life-threatening, inflammatory disease syndromes of the lung in which its function is critically compromised. ARDS is characterized by an acute onset of respiratory distress, edema formation, and severe hypoxemia1,2 associated with high mortality despite improved treatment regimen.3-5 The most common causes of ARDS are bacteria-derived pneumonia and sepsis.5-8 Despite efficient antibiotic therapy, inflammation often proceeds and leads to the breakdown of the endothelial barrier and immune-mediated injury of pulmonary tissue.9 The main drivers of this process are neutrophilic granulocytes (neutrophils), which infiltrate the tissue through a multistep process that requires diapedesis through the endothelium and the basement membrane of the blood vessel wall during the very early inflammatory phase.10-14 This rapid neutrophil recruitment to the lung vasculature peaks as early as after 4 hours in models of lipopolysaccharide (LPS)-induced ALI.15 Activated neutrophils release cytotoxic factors such as proteases, reactive oxygen species, and neutrophil extracellular traps (NETs) to combat pathogens.16 If this immune reaction is not tightly controlled spatio-temporally, it may cause the disruption of the alveolar–capillary barrier and formation of edema, leading to the loss of aerated tissue, severe hypoxemia, organ failure, and, ultimately, death.17,18 Accumulation and clustering of neutrophils at sites of tissue injury are classical hallmarks of acute inflammation.19,20 This neutrophil clustering has been observed in different murine tissues, including the skin, lymph nodes, and lung, during sterile tissue injury or infection with bacteria, fungi, or viruses,21-24 which may aggravate tissue damage.24 The molecular mechanisms underlying neutrophil recruitment, activation, and diapedesis are well studied,13 but the role of neutrophil cross talk with other cells during these processes is not fully understood.
Platelets are small, anucleate blood cells that play a key role in hemostasis and thrombosis.25,26 Besides this classical function, platelets are increasingly recognized as central orchestrators of inflammatory processes by supporting immune cell recruitment and activation as well as modulation of endothelial barrier function, thereby contributing to a pathomechanism referred to as thrombo-inflammation.27-29 Indeed, compelling experimental evidence suggests that in the pathogenesis of ALI and ARDS as well, neutrophil recruitment, activation, and formation of NETs (NETosis) are highly platelet dependent, and the formation of platelet–neutrophil complexes (PNCs) is considered to aggravate inflammation and the resultant pulmonary tissue damage.30-34 It has been implied that activated platelets promote these processes by direct or indirect interactions with neutrophils,33,35 and interference with this cross talk can reduce pulmonary inflammation.36,37 However, the contribution of initial platelet adhesion and activation pathways to neutrophil infiltration and activity in ALI has remained elusive.
Glycoprotein VI (GPVI) is a platelet-specific receptor for collagen and fibrin that mediates powerful platelet activation through a signaling cascade downstream of the receptor-associated immunoreceptor tyrosine-based activation motif (ITAM).38,39 Targeting of GPVI provides strong antithrombotic protection, with only minor impact on hemostasis.39-41 To our knowledge, a role for GPVI in thrombo-inflammation has not been assessed systematically. However, in models of glomerulonephritis, myocardial ischemia–reperfusion injury, and middle cerebral artery occlusion, lack of GPVI resulted in diminished inflammatory responses, but the underlying mechanisms have not been identified in detail.42-45 Similarly, in a model of pneumonia-derived sepsis, GPVI contributes to the pulmonary host defense and bacterial clearing,46 but how GPVI exerts this protective effect has remained unclear. In that study, replicating bacteria, which were not limited in growth by antibiotics, caused severe bacteremia. Under these conditions, the proliferating bacteria themselves mainly contribute to tissue damage, thereby masking a possible contribution of GPVI to thrombo-inflammatory neutrophil responses.
Here, we provide, to our knowledge, the first direct in vivo evidence that GPVI is required for efficient platelet–neutrophil interaction, neutrophil recruitment and cluster formation, and NETosis and that inhibition of GPVI function provides profound protection from neutrophil-mediated lung injury in a model of LPS-induced ALI.
Methods
Detailed methodology is provided in the supplemental Methods, available on the Blood website.
Animals
Experiments were carried out using 8- to 12-week-old male mice. C57BL/6J mice were supplied by Janvier (Le Genest, France). GPVI-deficient (Gp6−/−) mice have previously been described47 and were maintained in a specific pathogen-free environment and fed standard mouse diet ad libitum. Strain-, age-, and sex-matched wild-type (WT) mice were used as controls. Animal experiments were approved by the legal state authorities (Regierung von Unterfranken).
LPS-induced lung injury
Mice, under 2% isoflurane anesthesia, were intranasally instilled with LPS (Escherichia coli; O111:B4 serotype; Sigma Aldrich) at a dose of 5 mg/kg body weight (bw) to induce an acute alveolar inflammation.48,49 Sodium chloride (NaCl; 0.9%) served as vehicle control. After 4 hours, when neutrophil recruitment in the lung vasculature peaked,15 mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg bw) and xylazine (16 mg/kg bw). Blood was collected via the retro-orbital venous plexus for differential blood cell count (scil Vet abc Plus+, Horiba). Mice were euthanized via exsanguination, and a bronchoalveolar lavage was performed through 3 washes, each with 600 μL phosphate-buffered saline. The bronchoalveolar lavage fluid (BALF) was pooled, total cell counts were determined with a Neubauer improved hemocytometer, and cytospin preparations (Shandon Cytospin 4; Thermo Scientific) generated and stained with DiffQuick staining kit (HAEMA, LT-SYS; Labor+ Technik, Eberhard Lehmann GmbH) for differential cell counts.
Statistics
Statistical analyses were performed with GraphPad Prism version 9.0.0 (GraphPad Software). Data distribution was analyzed using the Shapiro-Wilk test, and differences were statistically analyzed using a 2-tailed Student t test or an ordinary one-way or a two-way analysis of variance (ANOVA) with a Šidák or a Tukey multiple comparisons test, as indicated in the figure legends. P values ≤ .05 were considered significant. Data were expressed as mean ± standard deviation.
Results
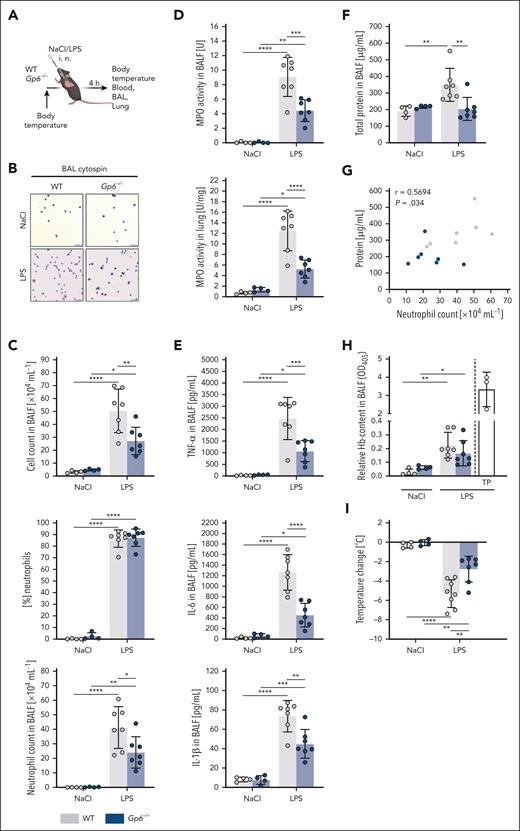
Diminished LPS-induced pulmonary inflammation and improved physiological outcome in Gp6−/− mice
To study a possible role of platelet GPVI in ALI, we treated WT and Gp6−/− mice intranasally with LPS (5 mg/kg bw; Figure 1A). After 4 hours, when the peak of vascular neutrophil recruitment was reached,15 alveolar cell recruitment was assessed via differential cell count using cytospin preparations of BALF (Figure 1B). LPS treatment caused a significant increase in leukocyte recruitment to the alveolar space in WT mice compared with that in vehicle-treated animals, and >85% of these intra-alveolar cells were neutrophils, as expected (Figure 1B-C). Notably, the LPS-induced neutrophil recruitment was strongly reduced in Gp6−/− mice, which was also reflected by a significantly reduced myeloperoxidase (MPO) activity in BALF as well as in whole lung lysates (Figure 1D). Compared with vehicle-treated mice, blood neutrophil counts were increased to a similar extent in both WT and Gp6−/− mice after LPS treatment (supplemental Figure 1A). Analysis of the BALF revealed markedly dampened levels of proinflammatory cytokines, notably, tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), and IL-1β in Gp6−/− animals after LPS treatment compared with those in WT mice (Figure 1E).
Gp6−/− mice show diminished LPS-induced pulmonary inflammation and physiological compromises. (A) Experimental scheme of LPS-induced ALI in C57BL/6J WT and Gp6−/− mice. LPS was administered at a dose of 5 mg/kg bw intranasally (IN). Sodium chloride (NaCl; 0.9%) was given as vehicle control. Samples were taken 4 hours after administration. (B) Representative micrographs of cytospin preparations of BALF cells stained with DiffQuick staining kit for differential cell counts. Images were acquired using a Thunder Imager DMi8 (Leica Microsystems) equipped with a 20× objective (HC PL APO 20×/0.80 DRY, Leica Microsystems) and a Leica-DMC4500-0265792920 camera (Leica Microsystems). Scale bar, 50 μm. (C) Quantification of total cell count and neutrophilic granulocytes in the BALF using a Neubauer improved hemocytometer and differential cell staining of cytospin preparations. (D) Spectrophotometric determination of MPO activity in the BALF cell pellet (top graph) and whole lung lysates (bottom graph). (E) Quantification of indicated cytokines (pg/mL) and (F) total protein content (μg/mL) in the cell-free BALF via DuoSet enzyme-linked immunosorbent assay kits (R&D Systems) and Pierce BCA Protein Assay Kit (Thermo Scientific), respectively. (G) Pearson correlation between total protein content (μg/mL) in the cell-free BALF and the neutrophil count in the BALF (×104 mL−1) of LPS-treated WT (gray) and Gp6−/− mice (blue). (H) Quantification of relative hemoglobin (Hb) content in the supernatant after erythrocyte lysis of the BALF cell pellet at 405 nm by spectrophotometry. (I) Determination of change in body temperature, 4 hours after administration of LPS or NaCl. Each data point represents 1 mouse. Bars represent mean ± standard deviation. The two-way analysis of variance (ANOVA) with a Šidák multiple comparisons test. ∗P ≤ .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. TP, thrombocytopenic.
Gp6−/− mice show diminished LPS-induced pulmonary inflammation and physiological compromises. (A) Experimental scheme of LPS-induced ALI in C57BL/6J WT and Gp6−/− mice. LPS was administered at a dose of 5 mg/kg bw intranasally (IN). Sodium chloride (NaCl; 0.9%) was given as vehicle control. Samples were taken 4 hours after administration. (B) Representative micrographs of cytospin preparations of BALF cells stained with DiffQuick staining kit for differential cell counts. Images were acquired using a Thunder Imager DMi8 (Leica Microsystems) equipped with a 20× objective (HC PL APO 20×/0.80 DRY, Leica Microsystems) and a Leica-DMC4500-0265792920 camera (Leica Microsystems). Scale bar, 50 μm. (C) Quantification of total cell count and neutrophilic granulocytes in the BALF using a Neubauer improved hemocytometer and differential cell staining of cytospin preparations. (D) Spectrophotometric determination of MPO activity in the BALF cell pellet (top graph) and whole lung lysates (bottom graph). (E) Quantification of indicated cytokines (pg/mL) and (F) total protein content (μg/mL) in the cell-free BALF via DuoSet enzyme-linked immunosorbent assay kits (R&D Systems) and Pierce BCA Protein Assay Kit (Thermo Scientific), respectively. (G) Pearson correlation between total protein content (μg/mL) in the cell-free BALF and the neutrophil count in the BALF (×104 mL−1) of LPS-treated WT (gray) and Gp6−/− mice (blue). (H) Quantification of relative hemoglobin (Hb) content in the supernatant after erythrocyte lysis of the BALF cell pellet at 405 nm by spectrophotometry. (I) Determination of change in body temperature, 4 hours after administration of LPS or NaCl. Each data point represents 1 mouse. Bars represent mean ± standard deviation. The two-way analysis of variance (ANOVA) with a Šidák multiple comparisons test. ∗P ≤ .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. TP, thrombocytopenic.
A critical step in the pathogenesis of ALI and ARDS is the loss of the capillary barrier function, causing edema formation, impeding gas exchange, and leading to hypoxemia.9,17 Although a significant increase in the total protein content in the BALF in response to LPS was detected in WT animals, this was not seen in Gp6−/− mice, indicating largely preserved alveolar–capillary barrier function (Figure 1F). Thereby, the protein concentration positively correlated with the number of intra-alveolar neutrophils (Figure 1G).
GPVI is critically required for the maintenance of vascular integrity under inflammatory conditions in some, but not all, tissues.50-52 We found that both WT and Gp6−/− mice developed comparable and rather minor intra-alveolar hemorrhages in response to LPS, confirming previous results50 (Figure 1H). In marked contrast, mice rendered severely thrombocytopenic via an injection of R300 antibodies (Emfret Analytics) showed severe pulmonary bleedings upon LPS challenge, confirming the established critical role of platelets in hemostasis in this setting. Most notably, Gp6−/− animals were in a significantly better overall condition than WT mice after LPS treatment and showed less severe physiological compromises, such as reduced activity or breathing rate. Moreover, although LPS-treated WT mice developed severe hypothermia, this response was markedly reduced in Gp6−/− animals (Figure 1I), indicating an improved physiological outcome.
These results showed that LPS-induced pulmonary inflammation and neutrophil infiltration are significantly reduced in the absence of GPVI, which translates into improved barrier function and physiological outcome.
Anti-GPVI treatment ameliorates LPS-induced pulmonary inflammation and edema formation
The aforementioned observations imply a strong impact of platelet GPVI on neutrophil recruitment into the LPS-challenged lungs. Thus, we sought to investigate whether the pharmacological targeting of GPVI reduces pulmonary inflammation in ALI. Therefore, WT mice were treated with nonimmune immunoglobulin G (control) or the anti-GPVI antibody JAQ1 (4 mg/kg bw), which induces the immunodepletion of the receptor, resulting in a sustained GPVI knockout–like phenotype.40 This induced loss of GPVI occurs in the liver and is associated with a transient, mild thrombocytopenia from days 1 to 3 after antibody injection.53 On day 5, when the JAQ1-treated mice displayed normal platelet counts and complete absence of GPVI in circulating platelets (supplemental Figure 2A-B), they were subjected to LPS-induced ALI (Figure 2A).
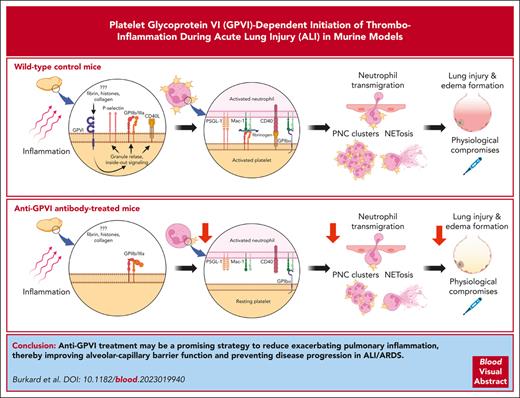
Anti-GPVI treatment ameliorates LPS-induced pulmonary inflammation and edema formation. (A) Experimental scheme of LPS-induced ALI in C57BL/6J WT mice treated with either anti-GPVI antibody JAQ1 or nonimmune immunoglobulin G (IgG; control) intraperitoneal (IP), 5 days before experiments. LPS was administered at a dose of 5 mg/kg bw IN. NaCl (0.9%) was given as vehicle control. Samples were collected after 4 hours. (B) Representative micrographs of cytospin preparations of BALF cells stained with DiffQuick staining kit for differential cell count. Images were acquired using a Thunder Imager DMi8 (Leica Microsystems) equipped with a 20× objective (HC PL APO 20×/0.80 DRY, Leica Microsystems) and a Leica-DMC4500-0265792920 camera (Leica Microsystems). Scale bar, 50 μm. (C) Quantification of total cell count and neutrophilic granulocytes in the BALF using a Neubauer improved hemocytometer and differential cell staining of cytospin preparations. (D) Spectrophotometric determination of MPO activity in the BALF cell pellet. (E) Quantification of the indicated cytokines (pg/mL) and (F) total protein content (μg/mL) in the cell-free BALF via DuoSet enzyme-linked immunosorbent assay kits (R&D Systems) and Pierce BCA Protein Assay kit (Thermo Scientific), respectively. (G) Pearson correlation between total protein content (μg/mL) in the cell-free BALF and the neutrophil count in the BALF (×104 mL−1) of LPS-treated control (gray) and JAQ1-administered mice (red). (H) Representative macroscopic images of perfused whole lungs of mice with indicated treatment after IV injection of 20 mg/kg bw Evans blue dye (EBD), 30 minutes before tissue sampling. Blue color shows extravascular EBD. Quantification of formamide-extracted EBD was performed via spectrophotometry against a standard curve and depicted as μg EBD per g lung tissue. (I) Quantification of relative Hb content in the supernatant after erythrocyte lysis of the BALF cell pellet at 405 nm via spectrophotometry. (J) Determination of the change in body temperature 4 hours after administration of LPS or NaCl. (K) Blood lactate levels were determined via a blood gas analyzer, using blood taken from the left ventricle. Each data point represents 1 mouse. Bars represent mean ± standard deviation. The two-way ANOVA with a Šidák multiple comparisons test. ∗P ≤ .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Anti-GPVI treatment ameliorates LPS-induced pulmonary inflammation and edema formation. (A) Experimental scheme of LPS-induced ALI in C57BL/6J WT mice treated with either anti-GPVI antibody JAQ1 or nonimmune immunoglobulin G (IgG; control) intraperitoneal (IP), 5 days before experiments. LPS was administered at a dose of 5 mg/kg bw IN. NaCl (0.9%) was given as vehicle control. Samples were collected after 4 hours. (B) Representative micrographs of cytospin preparations of BALF cells stained with DiffQuick staining kit for differential cell count. Images were acquired using a Thunder Imager DMi8 (Leica Microsystems) equipped with a 20× objective (HC PL APO 20×/0.80 DRY, Leica Microsystems) and a Leica-DMC4500-0265792920 camera (Leica Microsystems). Scale bar, 50 μm. (C) Quantification of total cell count and neutrophilic granulocytes in the BALF using a Neubauer improved hemocytometer and differential cell staining of cytospin preparations. (D) Spectrophotometric determination of MPO activity in the BALF cell pellet. (E) Quantification of the indicated cytokines (pg/mL) and (F) total protein content (μg/mL) in the cell-free BALF via DuoSet enzyme-linked immunosorbent assay kits (R&D Systems) and Pierce BCA Protein Assay kit (Thermo Scientific), respectively. (G) Pearson correlation between total protein content (μg/mL) in the cell-free BALF and the neutrophil count in the BALF (×104 mL−1) of LPS-treated control (gray) and JAQ1-administered mice (red). (H) Representative macroscopic images of perfused whole lungs of mice with indicated treatment after IV injection of 20 mg/kg bw Evans blue dye (EBD), 30 minutes before tissue sampling. Blue color shows extravascular EBD. Quantification of formamide-extracted EBD was performed via spectrophotometry against a standard curve and depicted as μg EBD per g lung tissue. (I) Quantification of relative Hb content in the supernatant after erythrocyte lysis of the BALF cell pellet at 405 nm via spectrophotometry. (J) Determination of the change in body temperature 4 hours after administration of LPS or NaCl. (K) Blood lactate levels were determined via a blood gas analyzer, using blood taken from the left ventricle. Each data point represents 1 mouse. Bars represent mean ± standard deviation. The two-way ANOVA with a Šidák multiple comparisons test. ∗P ≤ .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Similar to Gp6−/− animals, anti-GPVI–treated mice showed reduced overall leukocyte and neutrophil numbers as well as diminished MPO activity in the BALF in response to LPS compared with control mice (Figure 2B-D). Notably, anti-GPVI treatment had no effect on peripheral blood neutrophil counts in vehicle- or LPS-treated mice compared with that in corresponding controls (supplemental Figure 1B). After LPS treatment, the intra-alveolar release of the proinflammatory mediators TNF-α, IL-6, and IL-1β was reduced in anti-GPVI–treated animals compared with that in control animals (Figure 2E). In contrast, analysis of acute phase gene expression of Crp (encoding C-reactive protein), SAA2 (encoding serum amyloid A2), Lbp (encoding LPS-binding protein), and Cd14 (encoding CD14) in the liver showed no difference in control and anti-GPVI–treated mice 4 hours after LPS treatment, indicating that GPVI exerts its proinflammatory function locally at the site of acute inflammation but not systemically (supplemental Figure 3).
Compared with LPS-treated controls, JAQ1-treated mice were protected from LPS-induced alveolar edema formation as reflected by significantly reduced protein levels in the BALF (Figure 2F), which positively correlated with the number of infiltrated neutrophils (Figure 2G). Furthermore, interstitial edema formation was diminished in anti-GPVI–treated mice compared to controls after LPS treatment, as evidenced by reduced pulmonary Evans blue dye extravasation (Figure 2H). Like Gp6−/− mice, JAQ1-treated mice did not display any signs of increased inflammatory bleeding, and hemoglobin levels in the BALF remained at comparable low levels in both groups of animals after LPS challenge. These results were in contrast to the markedly increased hemoglobin content seen in the BALF of platelet-depleted mice in response to LPS (Figures 1H and 2I).
In agreement with the less severe pulmonary inflammation and reduced edema formation seen in anti-GPVI–treated mice, these mice developed a less-pronounced hypothermia in response to LPS compared to controls (Figure 2J). Moreover, the LPS-induced increase in blood lactate levels was significantly smaller in anti-GPVI–treated mice compared with that in controls (Figure 2K), showing that anti-GPVI treatment significantly improved the physiological outcome in this model of LPS-induced ALI.
Platelet GPVI is a critical mediator of neutrophil recruitment and transmigration
Because anti-GPVI treatment led to a less severe alveolar neutrophilia and improved physiological outcome after LPS treatment, we sought to investigate in more detail the mechanisms by which GPVI facilitates pulmonary inflammation.
Therefore, we performed multicolor immunofluorescence staining of lung cryosections. Imaging of whole left lung sections revealed marked and diffuse infiltration of neutrophils in control mice after LPS but not vehicle treatment (Figure 3A). This response was significantly less pronounced in JAQ1-treated mice, in which large areas more distal to the bigger airways showed similar neutrophil content as in vehicle-treated animals (Figure 3A). A detailed analysis with quantification of neutrophils in multiple fields of view (FOVs) at higher magnification showed significantly reduced overall neutrophil tissue infiltration in anti-GPVI–treated compared with that in control animals after LPS challenge (Figure 3B-C). This was supported by diminished MPO activity in whole lung lysates. Furthermore, in control mice, LPS instillation resulted in significantly higher numbers of platelets in the lung tissue than in vehicle-treated mice, whereas this increase was not found in anti-GPVI–treated mice (Figure 3B-D).
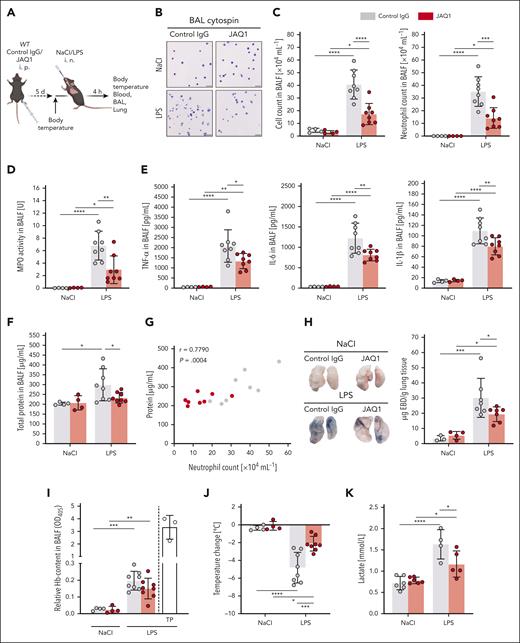
Platelet GPVI is a critical mediator of neutrophil recruitment and transmigration. Immunofluorescence staining of cryosections of the lungs of control or JAQ1-injected mice 4 hours after NaCl or LPS treatment. Images were acquired using a Thunder Imager DMi8 (Leica Microsystems) equipped with a 20× objective (HC PL APO 20×/0.80 DRY, Leica Microsystems) and a Leica-DFC9000GT-VSC12293 camera (Leica Microsystems). (A) Representative micrographs of whole left lung lobes showing extent of pulmonary neutrophil infiltration (Ly6G+, yellow). The dashed line depicts boundaries. Scale bar, 1 mm. (B) Magnified excerpts of a representative single FOV used to quantify neutrophils (Ly6G+, yellow) (left) and platelets (GPIX+, cyan) (right). Scale bar, 80 μm. (C-D) Quantification of neutrophil (C) (left graph) and platelet (D) numbers by manual counting. Neutrophils were identified as structures showing staining for 4′,6-diamidino-2-phenylindole (DAPI) (nucleus) and Ly6G. Spectrophotometric determination of MPO activity in whole lung lysates (C) (right graph). Each data point represents 1 mouse. (E) Representative confocal micrographs of neutrophils (Ly6G+, yellow), platelets (GPIX+, cyan), vessels (CD31+, magenta), and DAPI (nucleus, blue), exemplifying neutrophils counted as transmigrating (top) or intra-alveolar (bottom). Detailed representative images were acquired with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 63× objective (HC PL APO CS2 63×/1.40 OIL, Leica Microsystems). The dashed line marks the region of the magnified excerpt on the right. (Top) Scale bar, 10 μm; (bottom) scale bar, 30 μm. (F) Quantification of transmigrating neutrophils (left graph) by manual counting. Transmigrating neutrophils were identified as structures showing staining for DAPI (nucleus) and Ly6G, partially protruding into the AS. (G) Quantification of intra-alveolar neutrophils (left graph) by manual counting. Intra-alveolar neutrophils were identified as structures showing staining for DAPI (nucleus) and Ly6G, entirely inside the AS. Each data point represents the average number of neutrophils counted in 30 FOV for 1 mouse. Bars represent mean per group ± standard deviation. The two-way ANOVA with a Šidák multiple comparisons test. ∗P ≤ .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. AS, alveolar space.
Platelet GPVI is a critical mediator of neutrophil recruitment and transmigration. Immunofluorescence staining of cryosections of the lungs of control or JAQ1-injected mice 4 hours after NaCl or LPS treatment. Images were acquired using a Thunder Imager DMi8 (Leica Microsystems) equipped with a 20× objective (HC PL APO 20×/0.80 DRY, Leica Microsystems) and a Leica-DFC9000GT-VSC12293 camera (Leica Microsystems). (A) Representative micrographs of whole left lung lobes showing extent of pulmonary neutrophil infiltration (Ly6G+, yellow). The dashed line depicts boundaries. Scale bar, 1 mm. (B) Magnified excerpts of a representative single FOV used to quantify neutrophils (Ly6G+, yellow) (left) and platelets (GPIX+, cyan) (right). Scale bar, 80 μm. (C-D) Quantification of neutrophil (C) (left graph) and platelet (D) numbers by manual counting. Neutrophils were identified as structures showing staining for 4′,6-diamidino-2-phenylindole (DAPI) (nucleus) and Ly6G. Spectrophotometric determination of MPO activity in whole lung lysates (C) (right graph). Each data point represents 1 mouse. (E) Representative confocal micrographs of neutrophils (Ly6G+, yellow), platelets (GPIX+, cyan), vessels (CD31+, magenta), and DAPI (nucleus, blue), exemplifying neutrophils counted as transmigrating (top) or intra-alveolar (bottom). Detailed representative images were acquired with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 63× objective (HC PL APO CS2 63×/1.40 OIL, Leica Microsystems). The dashed line marks the region of the magnified excerpt on the right. (Top) Scale bar, 10 μm; (bottom) scale bar, 30 μm. (F) Quantification of transmigrating neutrophils (left graph) by manual counting. Transmigrating neutrophils were identified as structures showing staining for DAPI (nucleus) and Ly6G, partially protruding into the AS. (G) Quantification of intra-alveolar neutrophils (left graph) by manual counting. Intra-alveolar neutrophils were identified as structures showing staining for DAPI (nucleus) and Ly6G, entirely inside the AS. Each data point represents the average number of neutrophils counted in 30 FOV for 1 mouse. Bars represent mean per group ± standard deviation. The two-way ANOVA with a Šidák multiple comparisons test. ∗P ≤ .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. AS, alveolar space.
The number of neutrophils in the process of transmigration was increased in control animals after LPS challenge compared with that in vehicle-treated mice (Figure 3E,F). In contrast, transmigration was reduced by ∼76% in anti-GPVI–treated mice receiving LPS. Similarly, also the number of intra-alveolar neutrophils was decreased by ∼79% in JAQ1-treated mice compared with that in control mice (Figure 3E,G). These results were in line with the reduced number of neutrophils found in the BALF of anti-GPVI–treated mice compared with control mice after LPS challenge (Figure 2B). These findings show that GPVI is critically required for neutrophil extravasation in this setting. CD11b (αM-integrin) assembles with CD18 (β2-integrin) to form the adhesion receptor macrophage antigen 1 (Mac-1; αMβ2), which is essential for neutrophil transmigration.12 Hence, we measured the surface expression of CD11b on circulating neutrophils via flow cytometry and found lower CD11b expression levels on neutrophils of JAQ1-treated mice cells than on control cells, which may at least partly explain the reduced neutrophil transmigration in the lung (supplemental Figure 4).
GPVI mediates local platelet–neutrophil interactions
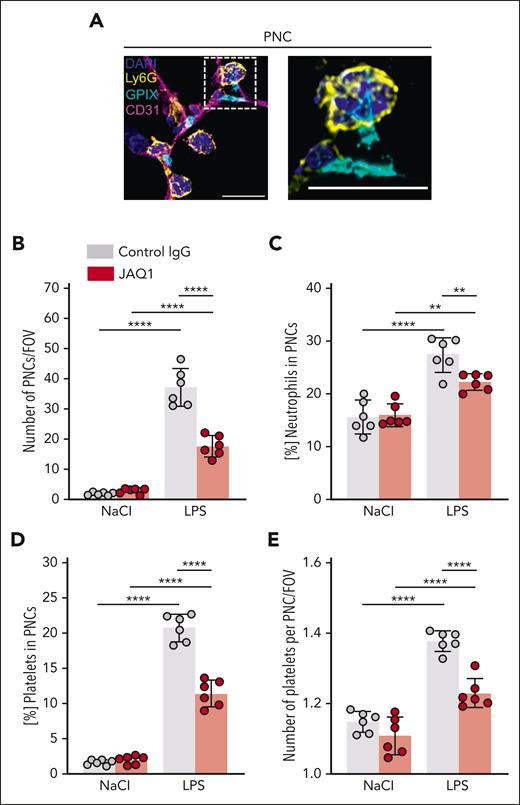
Platelets can modulate neutrophil behavior by direct interaction via multiple receptors, and inhibition of these interactions is reportedly protective in different inflammatory disease models.33,35,54 Thus, we quantified PNCs and found a marked increase in LPS-treated mice compared with that in vehicle-treated mice (Figure 4A), whereas this increase was significantly less in JAQ1-treated mice (Figure 4B). Of note, after LPS challenge, the ratio of neutrophils (Figure 4C) and platelets (Figure 4D) integrated in PNCs was significantly reduced in anti-GPVI–treated mice compared with that in control mice, indicating a role of GPVI in PNC formation in this setting. In line with this notion, we also found that the number of platelets per PNC after LPS challenge was reduced in anti-GPVI–treated mice compared with that in control mice (Figure 4E).
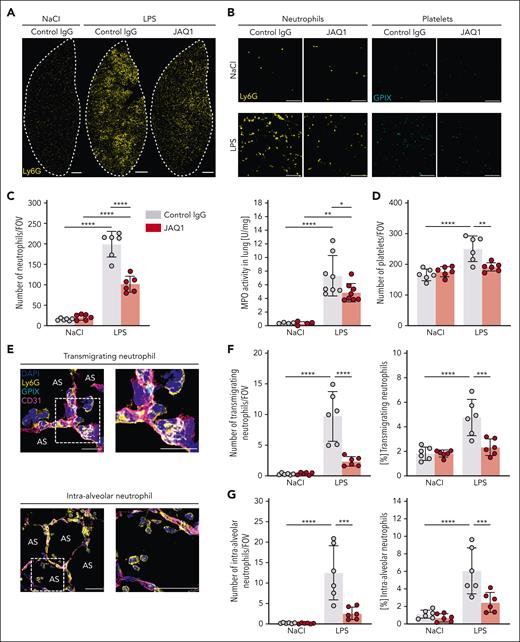
GPVI critically contributes to local platelet–neutrophil interactions in response to LPS. Immunofluorescence staining of cryosections of the lungs of control or JAQ1-injected mice, 4 hours after NaCl or LPS treatment. Images were acquired using a Thunder Imager DMi8 (Leica Microsystems) equipped with a 20× objective (HC PL APO 20×/0.80 DRY, Leica Microsystems) and a Leica-DFC9000GT-VSC12293 camera (Leica Microsystems). (A) Representative confocal micrographs of neutrophils (Ly6G+, yellow), platelets (GPIX+, cyan), vessels (CD31+, magenta), and DAPI (nucleus, blue), exemplifying neutrophils directly interacting with platelets to form PNCs. The dashed line marks the region of the magnified excerpt on the right without the vessel channel. Detailed representative images were acquired with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 63× objective (HC PL APO CS2 63×/1.40 OIL, Leica Microsystems). Scale bar, 10 μm. (B) Quantification of PNC numbers by manual counting. Each data point represents the average number of PNCs counted in 30 FOVs for 1 mouse. (C) Fraction of the total neutrophil number quantified in Figure 3C that was associated with platelets. Each data point represents mean value for 1 mouse. (D) Fraction of the total platelet number quantified in Figure 3D that was associated with neutrophils. Each data point represents mean value for 1 mouse. (E) Quantification of the number of platelets that associated with a neutrophil in 1 PNC. Each data point represents the average number of platelets that associated with a neutrophil in 1 PNC in 30 FOVs for 1 mouse. Bars represent mean per group ± standard deviation. The two-way ANOVA with a Šidák multiple comparisons test. ∗∗P < .01; ∗∗∗∗P < .0001.
GPVI critically contributes to local platelet–neutrophil interactions in response to LPS. Immunofluorescence staining of cryosections of the lungs of control or JAQ1-injected mice, 4 hours after NaCl or LPS treatment. Images were acquired using a Thunder Imager DMi8 (Leica Microsystems) equipped with a 20× objective (HC PL APO 20×/0.80 DRY, Leica Microsystems) and a Leica-DFC9000GT-VSC12293 camera (Leica Microsystems). (A) Representative confocal micrographs of neutrophils (Ly6G+, yellow), platelets (GPIX+, cyan), vessels (CD31+, magenta), and DAPI (nucleus, blue), exemplifying neutrophils directly interacting with platelets to form PNCs. The dashed line marks the region of the magnified excerpt on the right without the vessel channel. Detailed representative images were acquired with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 63× objective (HC PL APO CS2 63×/1.40 OIL, Leica Microsystems). Scale bar, 10 μm. (B) Quantification of PNC numbers by manual counting. Each data point represents the average number of PNCs counted in 30 FOVs for 1 mouse. (C) Fraction of the total neutrophil number quantified in Figure 3C that was associated with platelets. Each data point represents mean value for 1 mouse. (D) Fraction of the total platelet number quantified in Figure 3D that was associated with neutrophils. Each data point represents mean value for 1 mouse. (E) Quantification of the number of platelets that associated with a neutrophil in 1 PNC. Each data point represents the average number of platelets that associated with a neutrophil in 1 PNC in 30 FOVs for 1 mouse. Bars represent mean per group ± standard deviation. The two-way ANOVA with a Šidák multiple comparisons test. ∗∗P < .01; ∗∗∗∗P < .0001.
Intravital microscopy reveals reduced LPS-induced neutrophil sequestration and less stable platelet–neutrophil interactions in anti-GPVI–treated mice
In order to assess neutrophil behavior and investigate platelet–neutrophil interactions in response to LPS in more detail, we performed intravital confocal microscopy of the ventilated mouse lung (Figure 5A). Therefore, mice were treated with LPS 1 hour before imaging, anesthetized, and received fluorescently labeled antibody derivatives anti-GPIXAF546, anti-Ly6GAF488, and anti-CD31AF647 IV to stain for platelets, neutrophils, and endothelial cells, respectively. Mice were mechanically ventilated, and the left lung was immobilized, using a modified version of a thoracic suction window, as previously described.55 Images were taken every second, and the FOV was changed every 5 minutes. Image acquisition was performed for a total of 60 minutes, and videos of the time intervals from 0 to 5 minutes, 25 to 30 minutes, and 55 to 60 minutes were used for analyses. Determination of the area coverage of neutrophils and platelets revealed a marked reduction of both cell types in JAQ1-treated compared with control animals at all time intervals, recapitulating the findings of the immunofluorescence staining of lung cryosections (Figure 5B-C; supplemental Video 1). Neutrophil recruitment to the lung is a multistep process that involves the sequestration in the capillary bed, firm adhesion, crawling along the endothelium, and, eventually, emigration to the interstitial and alveolar space.12 In accordance with the reports by Yipp et al,56 we identified 3 categories of neutrophils: tethering, crawling, and adhering (Figure 5D). Automated tracking of neutrophils revealed that neutrophil adhesion increased over time in control but not in anti-GPVI–treated mice, which also displayed overall markedly decreased neutrophil adhesion compared with controls (Figure 5E). These findings were supported by an increased fraction of neutrophils that only briefly interacted with the vessel wall (tethering) in anti-GPVI–treated compared with control mice at all analyzed time intervals (Figure 5F), indicating impaired activation and/or upregulation of adhesion receptors of these cells. Of note, neutrophil crawling declined over time in both groups but was significantly reduced in anti-GPVI–treated mice compared with that in control mice (Figure 5G).
Intravital confocal microscopy reveals reduced LPS-induced neutrophil sequestration in anti-GPVI–treated mice. (A) Experimental scheme of the model of LPS-induced ALI in C57BL/6J WT mice treated with either 4 mg/kg bw anti-GPVI antibody JAQ1, or nonimmune IgG (control) IP, 5 days before intravital confocal microscopy. LPS was administered at a dose of 5 mg/kg bw IN. Imaging was performed with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 25× objective (HC FLUOTAR L 25×/0.95 WATER, Leica Microsystems) after 1 hour, and mice received fluorescently labeled antibody derivates to label neutrophils (Ly6G+, yellow), platelets (GPIX+, cyan), and vessels (CD31+, magenta). After 5 minutes, the FOV was changed. Total duration of imaging was 60 minutes. The time intervals from 0 to 5 minutes, 25 to 30 minutes, and 55 to 60 minutes were used for quantification. (B) Representative images derived from videos of the time interval from 25 to 30 minutes, illustrating the difference in neutrophil and platelet abundance in the 2 groups. Scale bar, 30 μm. (C) Quantification of the area (μm2) covered by the neutrophil (top graph) and platelet (bottom graph) fluorescence signal analyzed with Imaris Bitplane software at the indicated time intervals. (D) Automated tracking of neutrophils with Imaris Bitplane software. Representative images derived from videos of the time interval from 25 to 30 minutes showing probability masks of neutrophils superimposed with neutrophil tracks (left image) or the neutrophil tracks only (right image) of the indicated group. Blue tracks, adhesive neutrophils; red tracks, tethering neutrophils; and white tracks, crawling neutrophils. Scale bar, 30 μm. (E-G) Fraction of total neutrophils that were adhesive (E), tethering (F), and crawling (G). Each data point represents 1 mouse at the respective time interval. Bars represent mean time interval ± standard deviation. The two-way ANOVA with a Tukey multiple comparisons test. ∗P ≤ .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Intravital confocal microscopy reveals reduced LPS-induced neutrophil sequestration in anti-GPVI–treated mice. (A) Experimental scheme of the model of LPS-induced ALI in C57BL/6J WT mice treated with either 4 mg/kg bw anti-GPVI antibody JAQ1, or nonimmune IgG (control) IP, 5 days before intravital confocal microscopy. LPS was administered at a dose of 5 mg/kg bw IN. Imaging was performed with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 25× objective (HC FLUOTAR L 25×/0.95 WATER, Leica Microsystems) after 1 hour, and mice received fluorescently labeled antibody derivates to label neutrophils (Ly6G+, yellow), platelets (GPIX+, cyan), and vessels (CD31+, magenta). After 5 minutes, the FOV was changed. Total duration of imaging was 60 minutes. The time intervals from 0 to 5 minutes, 25 to 30 minutes, and 55 to 60 minutes were used for quantification. (B) Representative images derived from videos of the time interval from 25 to 30 minutes, illustrating the difference in neutrophil and platelet abundance in the 2 groups. Scale bar, 30 μm. (C) Quantification of the area (μm2) covered by the neutrophil (top graph) and platelet (bottom graph) fluorescence signal analyzed with Imaris Bitplane software at the indicated time intervals. (D) Automated tracking of neutrophils with Imaris Bitplane software. Representative images derived from videos of the time interval from 25 to 30 minutes showing probability masks of neutrophils superimposed with neutrophil tracks (left image) or the neutrophil tracks only (right image) of the indicated group. Blue tracks, adhesive neutrophils; red tracks, tethering neutrophils; and white tracks, crawling neutrophils. Scale bar, 30 μm. (E-G) Fraction of total neutrophils that were adhesive (E), tethering (F), and crawling (G). Each data point represents 1 mouse at the respective time interval. Bars represent mean time interval ± standard deviation. The two-way ANOVA with a Tukey multiple comparisons test. ∗P ≤ .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
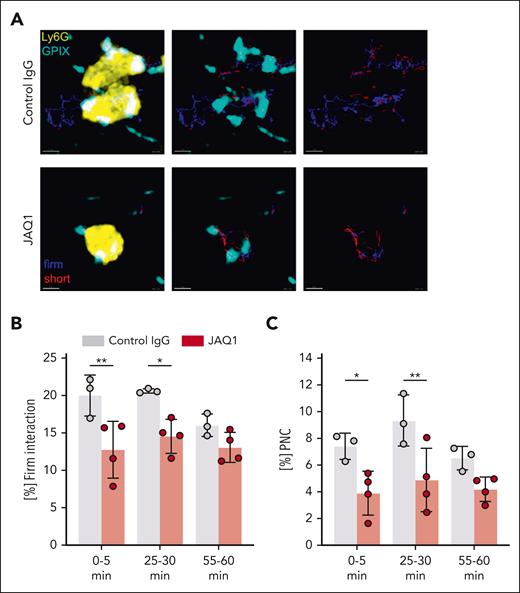
During intravital microscopy experiments, we noted frequent platelet–neutrophil interactions of different durations, ranging from very short “kiss-and-run”–like contacts to firm, stable interactions over several minutes (supplemental Video 2). Tracking of these interactions revealed that the proportion of platelets showing firm, long interactions (≥15 seconds) with neutrophils was decreased in anti-GPVI–treated mice compared with that in controls (Figure 6A-B), suggesting that these interactions were less stable in the absence of functional GPVI. Consequently, significantly less platelets interacted with neutrophils to form PNCs in anti-GPVI–treated mice compared with that in controls, which was also found in histological sections 1 hour after LPS treatment (Figure 6C; supplemental Figure 5). This direct interaction of neutrophils with platelets can support their recruitment to the inflamed tissue.30-34 The best characterized neutrophil-binding platelet receptors, such as P-selectin,57,58 CD40L,59 and activated GPIIb/IIIa,60 require platelet activation to be exposed, indicating that GPVI might trigger this activation rather than mediating the stable platelet binding to neutrophils. Because subendothelial collagens are not broadly exposed in the early thrombo-inflammatory phase of ALI, other ligands are likely to activate GPVI in this setting, and fibrin is 1 possible candidate. Immunofluorescence staining revealed that LPS treatment, indeed, led to pulmonary fibrin deposition, whereas no fibrin was detected in the lungs of vehicle-treated animals. Furthermore, pretreatment of mice with the potent thrombin inhibitor hirudin (5 μg/g bw61) abolished LPS-induced fibrin deposition (supplemental Figure 6) and resulted in significantly reduced neutrophil recruitment, NETosis, and diminished hypothermia (supplemental Figure 7A-D). These results, thus, support a possible role for fibrin as the GPVI ligand in this ALI model, but direct platelet activation by thrombin may also play a role. Remarkably, the lungs of the hirudin-treated mice did not show signs of major inflammatory bleeding compared with the control mice (supplemental Figure 7E), suggesting that thrombin/fibrin is not essential to maintain vascular integrity in this early thrombo-inflammatory phase of ALI. Of note, 5 μg/g bw hirudin treatment completely abolished FeCl3-induced arterial thrombosis, confirming its thrombin-inhibiting activity in vivo (supplemental Figure 7F).
Intravital confocal microscopy reveals impaired platelet–neutrophil interactions in anti-GPVI–treated mice after LPS treatment. (A) Automated tracking of platelets interacting with neutrophils with Imaris Bitplane software. Example images derived from videos of the time interval from 25 to 30 minutes, showing probability masks of neutrophils superimposed with platelet fluorescent signal and platelet tracks (left), or platelet fluorescence signal superimposed with platelet tracks (middle), or platelet tracks only (right) of the indicated group. Blue tracks, firm interacting platelet; and red tracks, short interacting platelets. Scale bar, 5 μm. (B-C) Fraction of interacting platelets that showed firm interaction (B) and fraction of total platelet tracks that showed firm interaction with neutrophils and are thus considered as PNC (C). Each data point represents 1 mouse at the respective time interval. Bars represent mean time interval ± standard deviation. The two-way ANOVA with a Tukey multiple comparisons test. ∗P ≤ .05; ∗∗P < .01.
Intravital confocal microscopy reveals impaired platelet–neutrophil interactions in anti-GPVI–treated mice after LPS treatment. (A) Automated tracking of platelets interacting with neutrophils with Imaris Bitplane software. Example images derived from videos of the time interval from 25 to 30 minutes, showing probability masks of neutrophils superimposed with platelet fluorescent signal and platelet tracks (left), or platelet fluorescence signal superimposed with platelet tracks (middle), or platelet tracks only (right) of the indicated group. Blue tracks, firm interacting platelet; and red tracks, short interacting platelets. Scale bar, 5 μm. (B-C) Fraction of interacting platelets that showed firm interaction (B) and fraction of total platelet tracks that showed firm interaction with neutrophils and are thus considered as PNC (C). Each data point represents 1 mouse at the respective time interval. Bars represent mean time interval ± standard deviation. The two-way ANOVA with a Tukey multiple comparisons test. ∗P ≤ .05; ∗∗P < .01.
Platelet GPVI contributes to local neutrophil activation
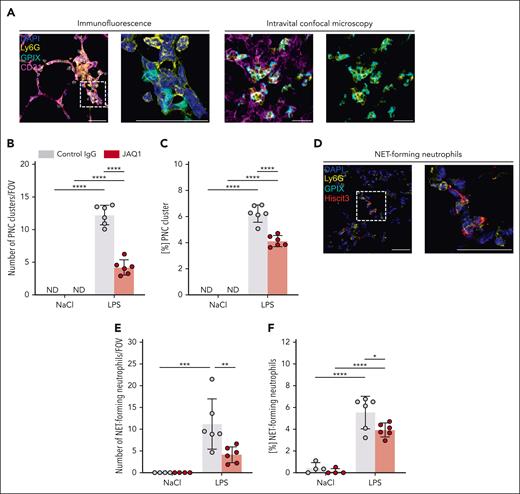
The reduced MPO activity in the BALF and whole lung lysates, the diminished adhesion and crawling of neutrophils in the capillary system, and the decreased platelet–neutrophil interactions suggested impaired activation of both cell types in the absence of functional GPVI. Furthermore, it is known that neutrophils can form larger clusters under acute inflammatory conditions and that this cluster formation can be detrimental for tissue integrity.24,62 Indeed, in response to LPS, we detected neutrophil clusters in histological sections but not in vehicle-treated mice (Figure 7A, left). Additionally, neutrophil cluster formation was observed in vivo by intravital confocal microscopy (Figure 7A, right; supplemental Video 3). Because platelets were frequently incorporated into those clusters, we termed them PNC clusters, which were markedly reduced in anti-GPVI–treated mice compared with in control mice (Figure 7B). Furthermore, the propensity of neutrophils to form PNC clusters was significantly reduced in anti-GPVI–treated mice compared with that in control mice (Figure 7C). This further indicated that the loss of functional GPVI resulted in reduced neutrophil activation. Thus, we stained histological sections for NETs (Figure 7D) and found a marked reduction of NET-forming neutrophils in anti-GPVI–treated mice compared with that in control mice after LPS challenge (Figure 7E-F). Together, these results demonstrated that functional GPVI is required for local neutrophil activation in this model of LPS-induced ALI.
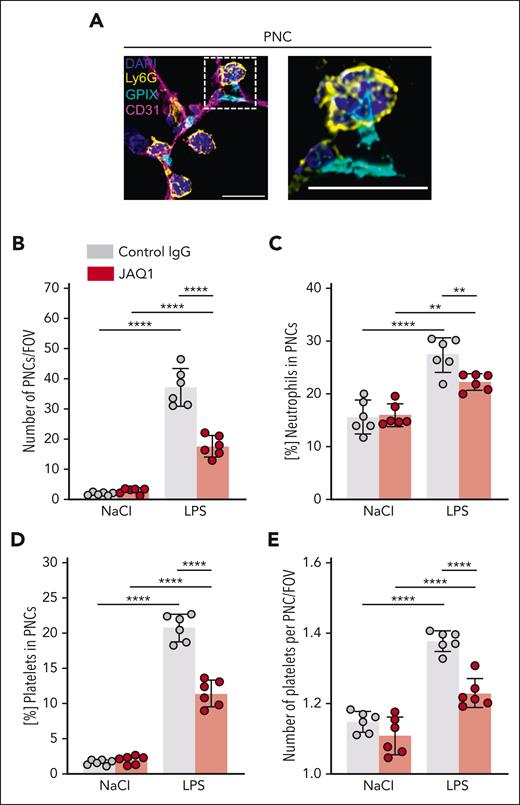
GPVI critically contributes to local neutrophil activation in response to LPS. (A) Representative confocal micrographs of neutrophils (Ly6G+, yellow), platelets (GPIX+, cyan), vessels (CD31+, magenta), and DAPI (nucleus, blue) exemplifying PNC cluster in immunofluorescence-stained cryosections of the lung (left) and during intravital confocal microscopy (right). The dashed line marks the region of the magnified excerpt on the right without the vessel channel. Detailed representative images of immunofluorescence-stained cryosections were acquired with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 63× objective (HC PL APO CS2 63×/1.40 OIL, Leica Microsystems). Intravital confocal microscopy was performed with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 25× objective (HC FLUOTAR L 25×/0.95 WATER, Leica Microsystems) after 1 hour of LPS treatment and mice received fluorescently labeled antibody derivates to label neutrophils, platelets, and vessels. Representative images derived from videos of the time interval 40 to 45 minutes showing accumulation of neutrophils as cluster with incorporated platelets. Scale bar, 30 μm. (B) Quantification of PNC clusters by manual counting in lung cryosections. Each data point represents the mean number of PNC clusters in 30 FOV for 1 mouse. (C) Fraction of neutrophils that formed PNC clusters. (D) Representative confocal micrographs of neutrophils (Ly6G+, yellow), platelets (GPIX+, cyan), NETs (Hiscit3+, red), and DAPI (nucleus, blue) exemplifying NET-forming neutrophils in immunofluorescence-stained cryosections of the lung. The dashed line marks the region of the magnified excerpt on the right without the vessel channel. Detailed representative images of immunofluorescence-stained cryosections were acquired with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 63× objective (HC PL APO CS2 63×/1.40 OIL, Leica Microsystems). Scale bar, 30 μm. (E) Quantification of NET-forming neutrophils by manual counting in lung cryosections. Each data point represents the mean number of NET-forming neutrophils in 30 FOV for 1 mouse. (F) Fraction of neutrophils that formed NETs. Each data point represents mean value for 1 mouse. Bars represent mean per group ± standard deviation. The two-way ANOVA with a Šidák multiple comparisons test. ∗P ≤ .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ND, not detectable.
GPVI critically contributes to local neutrophil activation in response to LPS. (A) Representative confocal micrographs of neutrophils (Ly6G+, yellow), platelets (GPIX+, cyan), vessels (CD31+, magenta), and DAPI (nucleus, blue) exemplifying PNC cluster in immunofluorescence-stained cryosections of the lung (left) and during intravital confocal microscopy (right). The dashed line marks the region of the magnified excerpt on the right without the vessel channel. Detailed representative images of immunofluorescence-stained cryosections were acquired with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 63× objective (HC PL APO CS2 63×/1.40 OIL, Leica Microsystems). Intravital confocal microscopy was performed with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 25× objective (HC FLUOTAR L 25×/0.95 WATER, Leica Microsystems) after 1 hour of LPS treatment and mice received fluorescently labeled antibody derivates to label neutrophils, platelets, and vessels. Representative images derived from videos of the time interval 40 to 45 minutes showing accumulation of neutrophils as cluster with incorporated platelets. Scale bar, 30 μm. (B) Quantification of PNC clusters by manual counting in lung cryosections. Each data point represents the mean number of PNC clusters in 30 FOV for 1 mouse. (C) Fraction of neutrophils that formed PNC clusters. (D) Representative confocal micrographs of neutrophils (Ly6G+, yellow), platelets (GPIX+, cyan), NETs (Hiscit3+, red), and DAPI (nucleus, blue) exemplifying NET-forming neutrophils in immunofluorescence-stained cryosections of the lung. The dashed line marks the region of the magnified excerpt on the right without the vessel channel. Detailed representative images of immunofluorescence-stained cryosections were acquired with a TCS-SP8 confocal laser scanning microscope (Leica Microsystems) with a 63× objective (HC PL APO CS2 63×/1.40 OIL, Leica Microsystems). Scale bar, 30 μm. (E) Quantification of NET-forming neutrophils by manual counting in lung cryosections. Each data point represents the mean number of NET-forming neutrophils in 30 FOV for 1 mouse. (F) Fraction of neutrophils that formed NETs. Each data point represents mean value for 1 mouse. Bars represent mean per group ± standard deviation. The two-way ANOVA with a Šidák multiple comparisons test. ∗P ≤ .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ND, not detectable.
Discussion
ALI and ARDS still account for high in-hospital mortality in clinics, especially among patients who are critically ill; however, to date, no fundamental pharmaceutical intervention exists.3-5 Pulmonary neutrophilia is a hallmark of ALI and ARDS and the main driver of tissue disruption.17,18 In this study we show that the loss of functional GPVI, by either genetic ablation or pharmacological targeting, markedly reduced neutrophil recruitment, activation, and extravasation and, thereby, ameliorated thrombo-inflammatory tissue damage and edema formation in a model of LPS-induced ALI in mice. Strikingly, the loss of functional GPVI had no effect on inflammatory bleeding under these conditions and significantly improved the physiological outcome.
Previous studies have shown a critical contribution of platelets to neutrophil recruitment in different models of acute inflammation, and PNC formation is considered a critical step in this process.30-34 Some of the platelet receptors involved in platelet–neutrophil interaction have been identified, and the most pathophysiologically relevant receptors require cellular activation to become functional. P-selectin and CD40L are surface exposed by degranulation and enable interaction with neutrophilic P-selectin glycoprotein ligand 157,58 and CD40,59 respectively, and GPIIb/IIIa requiring inside-out signaling to shift to a high affinity conformation in order to bind to neutrophilic Mac-1 (via fibrinogen).60 Experimental interference with these receptor–ligand interactions has been shown to reduce neutrophil recruitment, activation, and transmigration at sites of inflammation, thereby leading to a beneficial outcome.54,58,63,64 Moreover, the release of proinflammatory cytokines by activated platelets influences pulmonary neutrophil infiltration and neutralization of these cytokines reduced neutrophil recruitment and associated lung damage in a model of LPS-induced ALI.35
Although these molecular interactions are well defined, to our knowledge, the mechanisms of platelet activation and subsequent PNC formation in the onset of pulmonary inflammation were not identified. Our data demonstrate that GPVI plays a key role in this process, positioning this receptor at the very early steps of the (thrombo-)inflammatory cascade driving ALI/ARDS. Loss of functional GPVI resulted in diminished pulmonary PNC formation and reduced the tendency of platelets to interact with neutrophils as well as the time of interaction, suggesting that GPVI-dependent platelet activation contributes critically to platelet–neutrophil interactions in this setting. In the course of the inflammatory response, multiple GPVI ligands become accessible intravascularly, notably, histones,65 fibrin,66 and, with progressing tissue damage, also collagen, which further amplify platelet activation and resultant thrombo-inflammation.32 A possible role of fibrin as a GPVI agonist in this setting may be indirectly supported by the significant reduction in LPS-induced neutrophil recruitment, pulmonary NETosis, and protection from hypothermia as well as the absence of pulmonary fibrin deposition when mice were pretreated with hirudin (supplemental Figures 6C and 7A-D). However, it is clear that more detailed studies will be required to dissect the role of thrombin/fibrin, because thrombin is also a strong platelet activator and may, therefore, promote PNC formation directly through protease-activated receptor activation. Our results do, however, clearly exclude a major role of thrombin/fibrin for inflammatory hemostasis in the early thrombo-inflammatory phase of ALI (supplemental Figure 7E). Besides fibrin, the immunoglobulin superfamily receptor extracellular matrix metalloproteinase inducer (EMMPRIN),67,68 expressed on neutrophils, could contribute to GPVI activation in this setting, but further studies will be required to test this hypothesis.
Our data suggest that the inhibition of GPVI-mediated platelet activation in the setting of ALI simultaneously and efficiently prevents or reduces the exposure of multiple activation-dependent receptors for neutrophil interaction, thereby, strongly impairing platelet–neutrophil cross talk and resultant thrombo-inflammatory tissue injury. The observed marked reduction in platelet–neutrophil interactions indicates that other signaling pathways cannot fully compensate for the loss of GPVI-dependent platelet activation in this setting, particularly, thrombin, which does, however, have a role in this process, either directly or indirectly via fibrin (supplemental Figure 7A-D). A contribution of GPVI to tissue damage has previously been observed in other models of thrombo-inflammation, such as glomerulonephritis, myocardial ischemia/reperfusion injury, and acute ischemic stroke,42-44 but the underlying mechanisms were not addressed in detail. Here, we provide several lines of evidence that GPVI directly supports neutrophil recruitment, activation, and transmigration and, thereby, promotes local inflammation in the early thrombo-inflammatory phase of LPS-induced ALI. Studies using models of subacute lung injury triggered by a low dose of LPS (20 μg per mouse), focused on the role of GPVI in the prevention of inflammatory bleeding and found no effect of GPVI on neutrophil numbers at late time points.50,69 However, in this model, neither an exacerbating influx of neutrophils was triggered in the acute phase that would lead to the characteristic tissue damage seen in ALI/ARDS nor did the animals develop major physiological compromises within 4 hours (supplemental Figure 8A-C). Together with the late time point of analysis (24 hours), at which the peak of neutrophil recruitment has long passed15 and regenerative processes start, this might explain the (seemingly) contrary results on neutrophil recruitment observed in our study.
Another possible receptor–ligand pair for the initial contact of platelets and neutrophils is GPIbα/Mac-1.70 Indeed, Mac-1 (CD11b/CD18) is crucial for neutrophil sequestration and transmigration in the lung in response to LPS.12,71 Under inflammatory conditions, CD11b/CD18 is released from intracellular stores and upregulated on the surface of activated neutrophils, thereby, promoting neutrophil transmigration as well as the interaction with platelets.72,73 Yipp et al56 found CD11b to be essential for neutrophil crawling on the inflamed endothelium during endotoxemia. Our intravital microscopy studies on JAQ1-treated animals revealed reduced neutrophil sequestration and crawling, both of which are important prerequisites for transmigration,12,14,74 and reduced CD11b expression on circulating neutrophils. This led to markedly reduced neutrophil recruitment to the LPS-challenged lung and also a reduced propensity of the cells to transmigrate into the alveolar space.
Besides neutrophil extravasation, exacerbating neutrophil activation is causative for collateral tissue damage during ALI/ARDS and the disruption of the alveolar–capillary barrier.9,18 Thus, inhibition of excessive neutrophil activation might be beneficial for tissue integrity. Neutrophil cluster formation has been observed in various inflammatory settings such as sepsis24 or ALI75 and reported to promote neutrophil transmigration, activation, and tissue damage. In our model of LPS-induced ALI, neutrophils formed large clusters, in which platelets were frequently incorporated. This cluster formation was almost abrogated in the absence of functional GPVI. Furthermore, anti-GPVI treatment markedly reduced pulmonary NET formation in response to LPS. Similar results were obtained in a model of ventilator-induced lung injury, when the heterodimerization of the chemokines CCL5 and CXCL4, which are both released by activated platelets, was blocked.76 This shows that during the acute thrombo-inflammatory phase, disruption of GPVI-mediated ITAM-signaling is beneficial for tissue integrity. Contrary to this, in a murine model of sepsis, it has been shown that direct interaction of CD40L on platelets with CD40 on neutrophils can induce NETosis to promote bacterial clearance and improve long-term survival.59 Furthermore, the interaction of CD40L and CD40 on regulatory T cells is reportedly essential for the resolution of bacteria-derived pneumonia.77 Evidence that GPVI is the activatory receptor through which CD40L is upregulated in this setting is provided by another previous study showing that GPVI aids local host defense at later time points and that loss of GPVI increased bacterial loads while reducing circulating PNCs.46 In conjunction with our data, this shows that interference with GPVI during inflammation is a double-edged sword. During sepsis, thrombo-inflammatory processes that lead to excessive recruitment of neutrophils, which is promoted by platelet GPVI, characterize the very early phase. If the bacterial infection is not controlled by antibiotic treatment, as in the study of Claushuis et al,46 this can lead to severe bacteremia. In this situation, GPVI appears to be crucial in limiting bacterial spreading by promoting neutrophil effector function. Thus, the results of our study serve as a mechanistic explanation for the findings of Claushuis et al in their model of pneumosepsis, providing strong evidence for GPVI as an essential driver of the very early thrombo-inflammatory phase (ie, neutrophil recruitment, transmigration, and activation). If these steps are not tightly controlled, they may cause severe tissue damage independently of bacteremia and finally lead to ALI/ARDS.
In summary, we have shown that GPVI-mediated platelet activation critically contributes to neutrophil transmigration, PNC formation and, hence, neutrophil activation in LPS-induced ALI. Anti-GPVI treatment improved the alveolar–capillary barrier function in LPS-treated mice and mitigated physiological compromises. These results suggest that GPVI might be a promising target to limit the devastating pulmonary neutrophil influx and activation in the acute phase of ALI/ARDS without increasing the risk of inflammatory bleeding.
Acknowledgments
The authors thank the Core Unit Fluorescence Imaging of the Rudolf Virchow Zentrum (Center) for Integrative and Translational Bioimaging, University of Würzburg, for support with the data analysis.
This work was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation) Sonderforschungsbereich (SFB)/TR240, project 374031971 and SFB 1525, project 453989101.
Authorship
Contribution: P.B., B.N., and P.R. designed the study; P.B., C.S., D.J., and H.M.H. acquired all the data; D.K., L.T., P.R., A.Z., K.H., and K.G.H. helped with experimental setup; P.B., C.S., and H.M.H. analyzed the data; P.B., T.V., and B.N. drafted the manuscript; and all authors were involved in the interpretation of the data, critically reviewed and revised the manuscript for important intellectual content, and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Nieswandt, Institute of Experimental Biomedicine, University Hospital and Rudolf Virchow Center, University of Würzburg, Josef-Schneider-Str 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.
References
Author notes
Data are available on request from the corresponding author, Bernhard Nieswandt (bernhard.nieswandt@virchow.uni-wuerzburg.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.