Key Points
The newly identified AXL3 isoform is aberrantly expressed and represents a new biomarker and therapeutic target for MCL.
In a preclinical model of MCL, inhibition of AXL tyrosine kinase, using bemcentinib, shows superior activity to ibrutinib.
Abstract
Mantle cell lymphoma (MCL) is an aggressive B-cell non-Hodgkin lymphoma having a poor overall survival that is in need for the development of new therapeutics. In this study, we report the identification and expression of a new isoform splice variant of the tyrosine kinase receptor AXL in MCL cells. This new AXL isoform, called AXL3, lacks the ligand-binding domain of the commonly described AXL splice variants and is constitutively activated in MCL cells. Interestingly, functional characterization of AXL3, using CRISPR inhibition, revealed that only the knock down of this isoform leads to apoptosis of MCL cells. Importantly, pharmacological inhibition of AXL activity resulted in a significant decrease in the activation of well-known proproliferative and survival pathways activated in MCL cells (ie, β-catenin, Ak strain transforming, and NF-κB). Therapeutically, preclinical studies using a xenograft mouse model of MCL indicated that bemcentinib is more effective than ibrutinib in reducing the tumor burden and to increase the overall survival. Our study highlights the importance of a previously unidentified AXL splice variant in cancer and the potential of bemcentinib as a targeted therapy for MCL.
Introduction
Mantle cell lymphoma (MCL) is an aggressive type of B-cell non-Hodgkin lymphoma that originates from mature B cells. MCL represents between 5% to 7% of all non-Hodgkin lymphomas.1-5 Clinical management of MCL remains a significant challenge. Although current therapeutic options include several regimens of conventional chemotherapy, most patients will relapse, highlighting the need for more efficient molecularly targeted therapeutics specific for this disease. One of the characteristic features of MCL is the recurrent chromosomal translocation, t(11;14)(q13;q32) that brings the cyclin D1 gene under the control of the enhancer of the immunoglobulin heavy-chain gene, leading to the overexpression of the cyclin D1 protein, which in turn leads to a deregulation of the G1/S phase transition in the cell cycle.6 Abnormal overexpression of cyclin D1 was initially thought to be the primary driver of the disease. However, it was demonstrated that additional defects in many other cellular processes, such as those involved in cell survival and proliferation (WNT/β-catenin,7,8 NF-κB,9,10 Ak strain transforming (AKT),11 IL22RA1,12 apoptosis, DNA repair13), in addition to contributions from the tumor microenvironment14,15 are also required. More recently, the aberrant expression of the transcription factor SOX11 has been identified to be involved in the regulation of MCL cell growth.16,17 Furthermore, B-cell receptor expression, which is crucial for normal B-cell function, has been demonstrated to be constitutively activated in MCL development.18 Importantly, in phase 2 clinical trial, ibrutinib monotherapy targeting Bruton’s tyrosine kinase (BTK), which is constitutively activated in MCL, has been shown to achieve an overall response rate of 68% (n = 111) in patients with refractory/relapsed MCL.19 The median duration of response was ∼17.5 months.19,20 Clinical studies evaluating ibrutinib as a single agent21 or in combination with other agents, such as rituximab, resulted in an overall response rate of 87% (n = 50). More recently, emerging new therapies were investigated in MCL.22,23 The next generation of BTK inhibitors acalabrutinib, zanubrutinib, and pirtobrutinib have all been evaluated in MCL, with pirtobrutinib demonstrating encouraging results in refractory B-cell malignancies, including MCL.24 On the immunotherapy front, chimeric antigen receptor (CAR) T cells targeting CD19 have demonstrated durable remission in patients with relapsed/refractory MCL.25 Although these results highlight the potential of molecularly targeted approaches and immunotherapy (CAR T cells/Bi-specific T-cell engagers), those treatments are noncurative and, consequently, novel therapeutic modalities are still urgently required for this disease.20,26,27
The receptor tyrosine kinase AXL belongs to the Tyro3, Axl, MerTK (TAM) family of receptors.28,29 This small family of receptor tyrosine kinases shares the same overall structure and together they regulate and contribute to a wide array of signaling pathways leading to cellular responses such as survival, proliferation, and migration.30,31 The Human Protein Atlas32 reports that AXL is ubiquitously expressed in the human body but not detected in lymphoid tissue. Its expression in normal B cells is assumed to be absent at the messenger RNA (mRNA) and protein level.33,34 The vitamin K–dependent protein growth arrest–specific protein 6 (Gas6)35 is the principal ligand of AXL. The AXL receptor can also be activated through ligand-independent homodimerization and heterodimerization with a non-TAM receptor, such as EGFR.36 AXL signaling is transduced through PI3K-AKT-mTOR, MEK-ERK, NF-κB, and JAK/STAT activation.37 Interestingly, Axl was first discovered and cloned from chronic myeloid leukemia (CML) cells after blastic transformation, and identified as a transforming gene.28,29 AXL was described to have oncogenic activity in a variety of solid tumors (breast and lung cancer, melanoma, and glioma),28,31,34,37 and several forms of leukemia (acute myeloid leukemia [AML] and chronic lymphocytic leukemia).33,38 AXL expression was associated with poor prognosis and outcome in AML38 and has been demonstrated to be a driver of invasiveness, migration, and of therapeutic resistance in cancer cells.37 AXL has also been linked to the epithelial-to-mesenchymal transition in breast cancer and non–small cell lung carcinoma.37,39,40 Interestingly, although AXL expression has been investigated in most hematological malignancies,29 it has never been examined in MCL.
In this study, we investigate whether AXL could constitute a therapeutic target in MCL. We first demonstrate that AXL is expressed and activated in MCL. We then provide evidence for the existence of a third AXL isoform and its role in the survival of MCL cells. Moreover, we demonstrate the efficacy of bemcentinib, as a single agent or in combination, in a newly developed MCL xenograft model providing a translational basis for clinical implementation of AXL inhibition in MCL as a novel therapeutic strategy.
Methods
The study was performed in accordance with the Declaration of Helsinki, and all samples were collected after written informed consent. All patients were aged >16 years. The biobank and the clinical protocols were approved by the ethical committee at the Haukeland University Hospital, Bergen, Norway (ethical approval REK Vest 2012/2245) and Avicenne Hospital HUPSSD, Paris, France.
Details of procedures can be found in the supplemental Methods, available on the Blood website.
Cell culture and patient samples
The MCL cell lines (JeKo-1, SP53, REC-1, Mino, and Granta-519) previously described7 were used. The MCL cell line Maver-1 was a gift from Alberto Zamò (University of Verona, Verona, Italy). The CML cell line K562, and the ductal pancreatic adenocarcinoma cell line MIA-PaCa-2 cells were purchased from DSMZ (Braunschweig, Germany), whereas the Phoenix-AMPHO cells were kindly provided by BergenBio AS (Bergen, Norway).
Establishment of an inducible AXL shRNA knockdown system
To investigate the role of AXL in MCL, a short hairpin RNA (shRNA) conditional knockdown approach was used. The CML cell line K562 was used as a positive control. To transduce JeKo-1 and K562 cells with the vector of interest, retrovirus was made using Phoenix-AMPHO cells.
Generation of inducible sgRNA CRISPR inhibition cell lines
All plasmids used in the CRISPR inhibition (CRISPRi) experiment were purchased from Addgene (Watertown, MA) unless otherwise stated. JeKo-1 cells were first transduced with Lenti-dCas9-KRAB-blast (plasmid #89567). dCas9 positivity was confirmed by western blotting, as previously described, using an anti-Cas9 monoclonal antibody (Prod nr. 10C11-A12, ThermoFisher Scientific). Positive cells were then transduced with a FgHtUTG (plasmid #70183) plasmid cloned with single guide RNA (sgRNA) sequences targeting AXL3. The cells were named JeKo-1 dCas9 sgRNA1, JeKo-1 dCas9 sgRNA2, and JeKo-1 dCas9 sgRNA3.
sgRNA design and cloning into the inducible sgRNA expression vector
The CRISPRi sgRNA sequences were designed using the designer tool CRISPR-ERA version 1.2, using an input of 1500 base pairs (bp) upstream of the transcription start site and 1500 bp downstream of the transcription start site of the AXL3 transcript (http://www.ncbi.nlm.nih.gov/).
In vivo evaluation of bemcentinib in an MCL mouse xenograft model
The lentiviruses, RediFect Red-FLuc-GFP, were purchased from PerkinElmer Inc (Waltham, MA) and used to transduce the JeKo-1 cells and generate the JeKo-1Luc+ cells that contain green fluorescence protein and luciferase reporter. All the animal experimentations were approved by the Norwegian Animal Research Authority and performed in accordance with the European Convention for the protection of vertebrates used for scientific purposes.
Results
AXL is expressed in MCL cell lines and primary MCL cells
To investigate the presence of AXL in MCL, we first demonstrated the expression of AXL transcript using reverse transcription polymerase chain reaction. Primers were specifically designed to amplify AXL isoform 1 and 3 based on the exon structure released in a public database (National Center for Biotechnology Information) (Figure 1A; supplemental Figure 1A). However, because the AXL isoform 2 differs only by a 27-bp internal exon, the primer set named AXL1-2 is also able to amplify AXL isoform 1 and 2. RNA extracted from 5 MCL cell lines was used, and the results are illustrated in Figure 1B. AXL mRNA was detectable in the MCL cell lines examined. MIA-PaCa-2 and K562 cells served as positive controls. We detected AXL protein expression in MCL cell lines using western blotting. Because AXL is a heavily glycosylated protein, we used 2 different antibodies with distinct binding sites to avoid false negative detection of AXL. One antibody targeting the N-terminal part of the protein (labeled N-terminal) and the other antibody recognizing the C-terminal part of the AXL protein (labeled C-terminal). As shown in Figure 1C, the C-terminal antibody can recognize AXL at its expected molecular weight in MIA-PaCa-2 cells (∼98 kDa), as well as the glycosylated form at 120 to 140 kDa. However, in all MCL cells, this antibody recognizes a band corresponding to the expected size of AXL3 (∼70 kDa). This isoform can also be detected in K562 and MIA-PaCa-2 but at a lower level of expression than in MCL cells. In contrast, the AXL N-terminal antibody can identify AXL expression at a size of ∼120 to 140 kDa in all the cell lines tested (Figure 1D). Flow cytometry was used to evaluate the expression of AXL in MCL cell lines. As demonstrated in Figure 1E, the Jeko-1 MCL cell line showed an increased signal in comparison with the isotype control. K562 cells served as the positive control in this experiment. We then assessed the expression of AXL in patient–derived primary MCL cells at both mRNA and protein level. As shown in Figure 1F, the different AXL transcripts can be detected in samples from patients with MCL. Furthermore, using either the AXL N-terminal or C-terminal antibodies, AXL protein expression can be detected in MCL primary cells (Figure 1G-H) and is overexpressed in comparison with peripheral blood mononuclear cells or CD19+ cells. To confirm that MCL expresses the AXL3 transcript, we cloned the full complementary DNA transcript including the 5′ and 3′ untranslated regions (UTRs) from JeKo-1 and K562 cells (Ensemble database: putative AXL-203 transcript ID ENST00000593513.1; 2557 bp). As shown in Figure 1I, the amplification product using the primer to clone the AXL3 transcript (supplemental Figure 1B) led to the amplification of products at the expected size. The full product was cloned, and the alignment of the sequencing result confirmed the presence and identity of the AXL3 transcript with the predicted exon structure (Figure 1J-K). We also cloned part of the AXL1 mRNA excluding a portion of the 3′ UTR. The full amplification product was also cloned and confirmed the existence of AXL1 transcript in MCL cells (Ensemble database: AXL-201 transcript ID ENST00000301178.9; 4717 bp).
AXL is constitutively activated in MCL cell lines and primary MCL cells
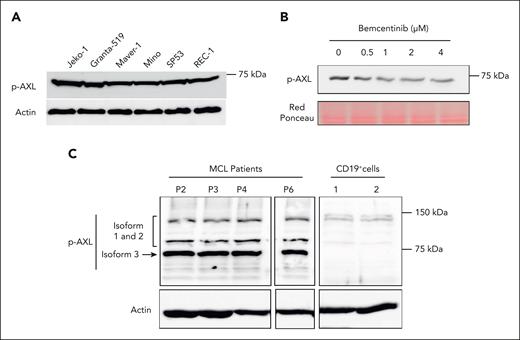
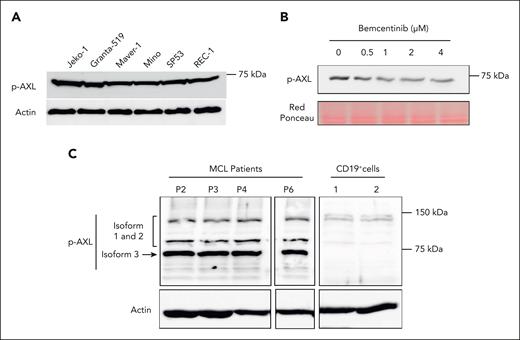
To investigate the level of AXL activation in MCL cell lines and MCL primary cells, we examined its phosphorylation status by western blot using an antibody specific for phosphorylated AXL (p-AXL). As shown in Figure 2A, AXL phosphorylation can be detected in all MCL cell lines with bands corresponding to the expected molecular weight of AXL3. To confirm the specificity of the detected AXL phosphorylation, the MCL cell line JeKo-1 was treated with increasing doses of the AXL inhibitor, bemcentinib. As described in Figure 2B, the AXL inhibitor induced a significant decrease in the phosphorylation status of AXL. AXL phosphorylation was also evaluated in patient–derived MCL primary cells (Figure 2C), demonstrating AXL phosphorylation. Both in MCL cell lines and patient cells, the strongest AXL phosphorylation was detected for the band corresponding to the third isoform of AXL. CD19+ cells demonstrated some weak p-AXL staining for AXL isoform 1 to 2 in comparison with MCL cells (Figure 2C). We also evaluated the expression of GAS6, the ligand of AXL, in MCL cells. As shown in supplemental Figure 2A, the GAS6 transcript can be detected in all MCL cell lines. However, no GAS6 protein expression can be detected in the supernatant of MCL cell-culture medium (supplemental Figure 2B). Together, these observations indicate that the newly described AXL3 protein is the major AXL isoform activated in MCL cells, and this is supported by the observation that the known ligand of AXL is not expressed at the protein level in MCL cell lines, and no p-AXL1 and p-AXL2 isoforms could be detected.
AXL is constitutively activated in MCL cell lines and patient cells. (A) Western blot studies demonstrated the phosphorylation of AXL in all MCL cell lines. (B) JeKo-1 cells were treated with bemcentinib at concentrations ranging from 0.5 to 4 μM. Phosphorylation of AXL was evaluated using a p-AXL antibody. Bemcentinib treatment reduces the level of AXL phosphorylation in MCL cells. (C) AXL phosphorylation status was evaluated in cells from patients with MCL by western blot. The AXL phosphorylation was detectable in all patient–derived MCL cells. The results also indicated that AXL3 is the isoform predominantly activated in primary MCL cells. In contrast, CD19+ B cells from healthy individuals present a weak AXL activation and the complete absence of AXL3 phosphorylation. Experiments were performed in triplicate.
AXL is constitutively activated in MCL cell lines and patient cells. (A) Western blot studies demonstrated the phosphorylation of AXL in all MCL cell lines. (B) JeKo-1 cells were treated with bemcentinib at concentrations ranging from 0.5 to 4 μM. Phosphorylation of AXL was evaluated using a p-AXL antibody. Bemcentinib treatment reduces the level of AXL phosphorylation in MCL cells. (C) AXL phosphorylation status was evaluated in cells from patients with MCL by western blot. The AXL phosphorylation was detectable in all patient–derived MCL cells. The results also indicated that AXL3 is the isoform predominantly activated in primary MCL cells. In contrast, CD19+ B cells from healthy individuals present a weak AXL activation and the complete absence of AXL3 phosphorylation. Experiments were performed in triplicate.
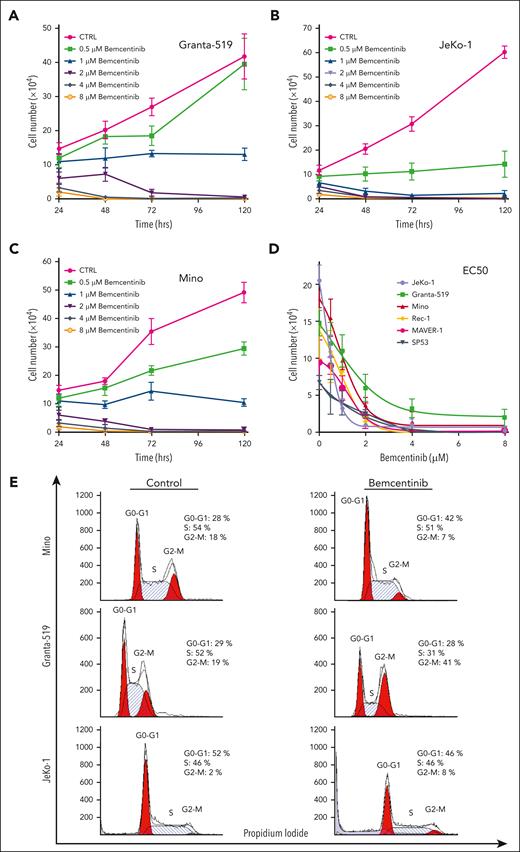
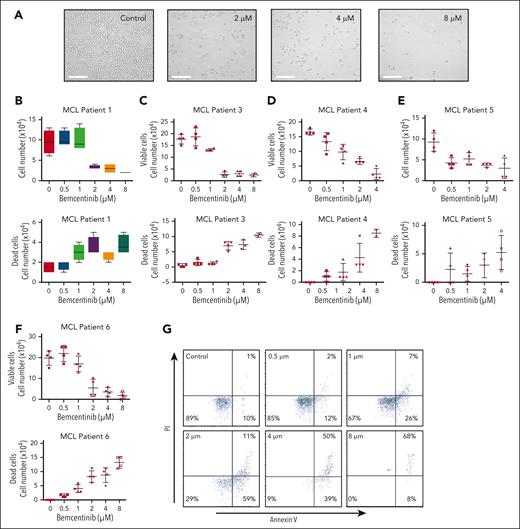
Pharmacologic inhibition of AXL activity by bemcentinib induces significant cell growth inhibition and apoptosis of MCL cell lines
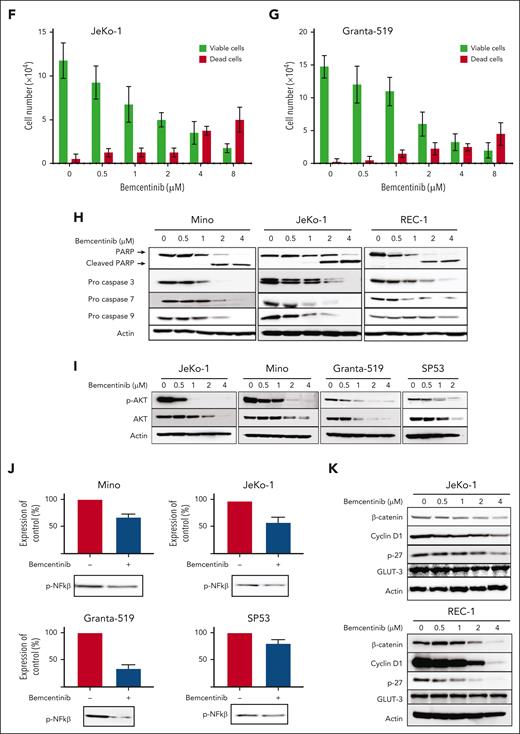
To determine the biological importance of AXL in MCL cells, we inhibited AXL activation in MCL cell lines using the most potent pharmacological inhibitor described so far, bemcentinib.41 To show that bemcentinib can bind AXL in vivo we used the cellular thermal shift assay (supplemental Figure 3). To determine the 50% effective concentration (EC50) of bemcentinib in MCL cell lines, the MCL cells were treated with a wide range of drug concentrations. As shown in Figure 3, bemcentinib induced a significant reduction in the number of cells in a time- and dose-dependent manner (Figure 3A-C). At 48 hours after treatment, the EC50 values were in the range of 1 to 2 μM for most of the MCL cell lines (Figure 3D) with SP53 being the most sensitive cell line and Maver-1 the least sensitive (Figure 3D). As illustrated in Figure 3E, cell cycle analysis revealed that bemcentinib induces cell cycle arrest either in the G1 phase, for the Mino cells, or at the G2 phase for the Granta-519 and JeKo-1 MCL cells. Next, we investigated whether bemcentinib induced apoptosis in MCL cells. As shown in Figure 3F-G, bemcentinib treatment of various MCL cell lines for 48 hours leads to an increase in trypan blue–positive cells. Moreover, a dose-dependent cleavage of poly adenosine diphosphate ribose polymerase (PARP), as well as proteolytic activation of procaspases 3, 7, and 9 can be observed after bemcentinib treatment (Figure 3H). We also found that increasing concentrations of bemcentinib induced a dose-dependent decrease in AKT (Figure 3I) and NF-κB activation (Figure 3J). Furthermore, we also evaluated the biological effect of bemcentinib on other pathways known to be dysregulated in MCL. As shown in Figure 3K, bemcentinib induced a decrease in the expression of cyclin D1, p27, and of β-catenin. Taken together, these results demonstrate that major signaling pathways involved in the survival and proliferation of MCL cells are inhibited by the inhibition of AXL activation.
Knockdown of the AXL 3 isoform induces apoptosis of MCL cells
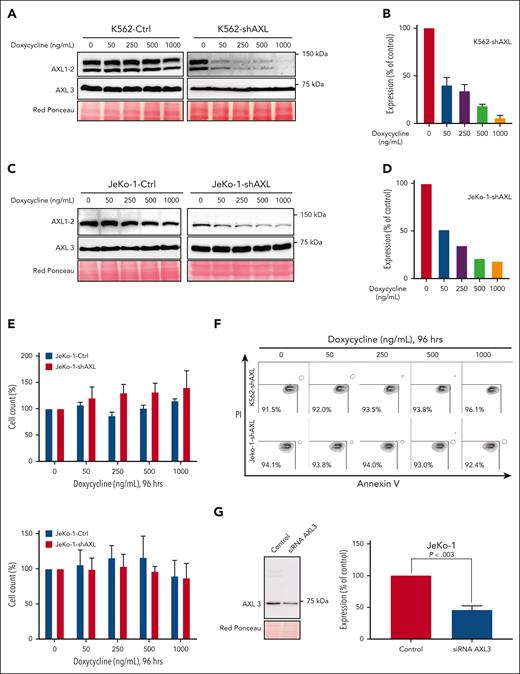
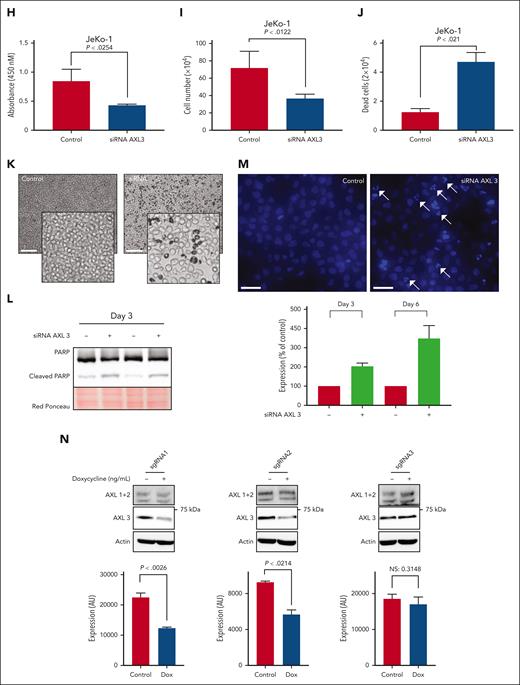
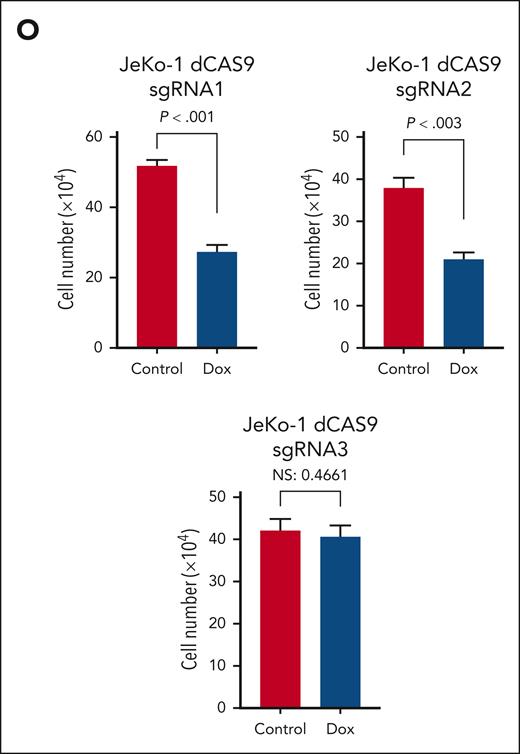
To further investigate the role of AXL in MCL, we used a conditional shRNA approach. The MCL cell line JeKo-1 and the CML cell line K562 were transduced with the inducible AXL shRNA or control shRNA vector containing the mCherry reporter vector. As shown in supplemental Figure 4A-B, an mCherry fluorescence signal, indicating the presence of the inducible construct, is observed in the different transduced cell lines. AXL knockdown was induced by adding different concentrations of doxycycline (ranging from 0 to 1000 nM; Figure 4A,C). The knockdown efficiency was analyzed by western blotting, and a 90% decrease of AXL1 and AXL2 can be observed in the 2 different models (Figure 4B,D; supplemental Figure 4C-D). In contrast, no decrease in AXL protein was observed in the control cells (Figure 4A,C, left panel). Interestingly, the level of AXL3 expression is not affected by the increased concentrations of doxycycline in the K562 transduced cell line. Similarly, a decrease in protein expression of AXL1 and AXL2 can be observed in JeKo-1-shAXL cells after addition of doxycycline. However, as seen in the K562 cells, AXL3 expression remains unaltered. To evaluate the effect of decreased AXL expression on cell growth and viability we used the trypan blue assay. Surprisingly, no statistical difference in total cell number was observed between the different doxycycline concentrations in any of the transduced cell lines (Figure 4E). Moreover, the control and the shRNA-AXL cell lines present no statistical difference in the total cell number after 96 hours of doxycycline treatment. Annexin V/propidium iodide flow cytometry was performed to further evaluate the impact of AXL1 and AXL2 knockdown on cell viability. After 96 hours of doxycycline treatment, cells were stained and analyzed using flow cytometry and no statistical differences in the number of apoptotic cells were observed for the different concentrations of doxycycline used (Figure 4F). All cell lines were ∼90% viable after 96 hours exposure to 1000 nM doxycycline. To further evaluate the biological significance of AXL in MCL, we investigated the role of AXL3 on MCL biology. AXL3 expression was downregulated using a small-interfering RNA (siRNA) approach and we assessed the effects on cell growth and apoptosis on MCL cells. As shown in Figure 4G, siRNA specifically targeting AXL3 (supplemental Figure 5A) induced a substantial reduction in AXL3 protein expression of ∼50% within 72 hours of transfection. Blockade of AXL3 expression using siRNA significantly decreased cell growth by ∼50% at 5 days after transfection as determined by WST-1 assay (Figure 4H) and cell count (Figure 4I; supplemental Figure 5B). Moreover, bright-field optical microscope imaging revealed the presence of dead cells in cells transfected with the AXL3 siRNA (Figure 4J-K). As shown in Figure 4L, inhibition of AXL3 expression by siRNA in JeKo-1 cells induced the cleavage of PARP. Moreover, Hoechst staining (Figure 4M) clearly demonstrated formation of apoptotic bodies with condensed and fragmented nuclei. These results underline that, in contrast to the AXL1 and 2 isoforms, only the specific downregulation of AXL3 protein expression has an impact on MCL cell growth and viability. Because the design of specific AXL3 siRNA needed to target the 5′ UTR sequence of the AXL3 mRNA, which prevented us finding multiple suitable effective siRNA sequences, we decided to validate our siRNA observations using an CRISPRi approach. To this end, JeKo-1 cells were made positive for dCAS9 expression (supplemental Figure 6A-C) and we designed 3 sgRNAs targeting the AXL3 promoter (supplemental Figure 6D). As shown in Figure 4N (left and middle panel), 2 of the sgRNAs targeting the AXL3 lead to a decrease in AXL3 protein expression, which was accompanied by a decrease in cell proliferation (Figure 4O). In contrast, no effect on cell proliferation was observed in control cells (supplemental Figure 6E) or cells carrying the sgRNA that failed to decrease AXL3 expression (Figure 4N-O, right panel).
Bemcentinib induces cells death of primary MCL cells in vitro
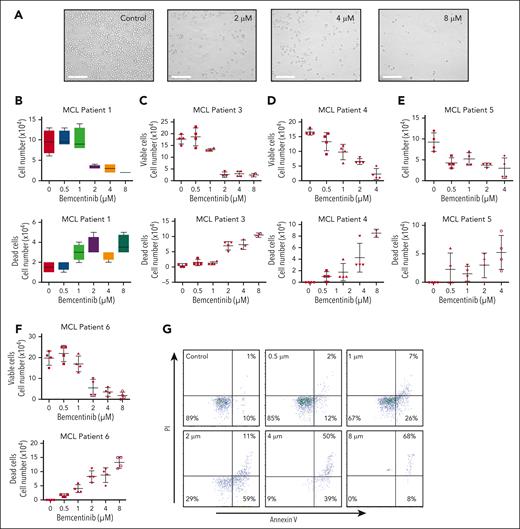
To further evaluate the efficacy of bemcentinib on MCL cells, we treated primary MCL cells with various concentrations of bemcentinib (Figure 5A). Bemcentinib induced a significant dose-dependent reduction in cell growth, as well as a decrease in cell viability, as determined by trypan blue staining (Figure 5B-F). This result is further supported by annexin V/propidium iodide staining, which shows an increase in cell death after treatment with bemcentinib (Figure 5G). As observed for the MCL cell lines, the EC50 of bemcentinib is ∼1.5 μM in MCL primary cells (Figure 5B-F). Moreover, we also demonstrate synergy between bemcentinib and ibrutinib in vitro (supplemental Figure 7). Overall, these results indicate that MCL primary cells are sensitive to bemcentinib in vitro.
Bemcentinib induces apoptosis of MCL primary cells. (A) Patient–derived MCL cells were treated with dimethyl sulfoxide (DMSO) or various concentrations of bemcentinib (0-8 μM) for 24 hours. Relevant microscopy pictures revealed the presence of apoptotic cells and a reduction of the cell populations. Scale bar, 100 μM. (B-F) Cell proliferation and cell death were measured by cell counting and trypan blue staining. Experiments were performed 4 times and the mean and distribution are shown. (G) Cell death was also investigated by flow cytometry using annexin V/PI staining in patient–derived MCL cells. Bemcentinib induced apoptosis in primary MCL cells in vitro.
Bemcentinib induces apoptosis of MCL primary cells. (A) Patient–derived MCL cells were treated with dimethyl sulfoxide (DMSO) or various concentrations of bemcentinib (0-8 μM) for 24 hours. Relevant microscopy pictures revealed the presence of apoptotic cells and a reduction of the cell populations. Scale bar, 100 μM. (B-F) Cell proliferation and cell death were measured by cell counting and trypan blue staining. Experiments were performed 4 times and the mean and distribution are shown. (G) Cell death was also investigated by flow cytometry using annexin V/PI staining in patient–derived MCL cells. Bemcentinib induced apoptosis in primary MCL cells in vitro.
Bemcentinib shows superior efficacy over ibrutinib to decrease MCL tumor burden and enhances overall survival
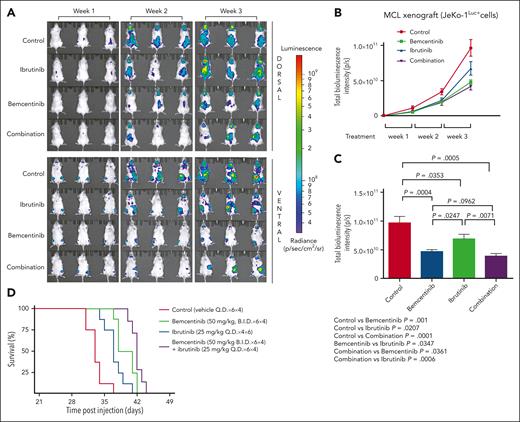
To investigate whether pharmacological targeting of AXL could potentiality benefit patients, we used our newly developed orthotopic JeKo-1 xenograft mouse model of MCL (supplemental Figure 8A-F). The in vivo efficacy of bemcentinib, ibrutinib, and the combination of the 2 drugs was evaluated. As shown in Figure 6A, the disease develops in the bone marrow, spleen, femur, and brain. Bioluminescence imaging revealed that at week 3 the control group had the highest average total bioluminescence signal, followed by the ibrutinib treatment group and the bemcentinib treatment group, respectively (Figure 6B-C). The bemcentinib treatment group demonstrated significantly lower total bioluminescence signal compared with the control group (P = .0004; unpaired Student t test), whereas the ibrutinib treatment group also showed statistically significant decrease in total bioluminescence in comparison with the control group (P = .0353; unpaired Student t test). In addition, the total bioluminescence signal of the bemcentinib treatment group was significantly lower than that of the ibrutinib treatment group (P = .0247; unpaired Student t test). The total bioluminescence of the combination group was also significantly lower than that of the control (P = .0005; unpaired Student t test) and the ibrutinib group (P = .0071; unpaired Student t test). These results demonstrate that bemcentinib shows efficacy in a preclinical xenograft MCL mouse model. The combination of bemcentinib and ibrutinib demonstrates the highest overall survival (Figure 6D).
Inhibition of AXL by bemcentinib reduces tumor burden and increases survival in an MCL xenograft mouse model. (A) Female nonobese diabetic severe combined immunodeficiency gamma mice were injected IV with 5 × 105 JeKo-1Luc+ cells. The mice were imaged weekly, both dorsally and ventrally (10 minutes after intraperitoneal injection with 150 mg/kg of 25 mg/mL D-luciferin). The mice were distributed in the following groups: control (vehicle only every day), bemcentinib treatment (50 mg/kg twice a day), ibrutinib treatment (25 mg/kg every day), and combination treatment (ibrutinib + bemcentinib) based on total bioluminescence. Following the assignment, there was no statistical difference between the groups (P > .99; 1-way analysis of variance test). JeKo-1Luc+ xenografts were treated for 4 weeks with vehicle every day (control), ibrutinib (25 mg/kg, every day), bemcentinib (50 mg/kg, twice a day) or the combination of ibrutinib and bemcentinib (same dose as single treatment) (n = 6). (B) Bioluminescence imaging (BLI) was assessed weekly to monitor disease progression. (C) Quantification of BLI signal from the different treatment groups in week 3 (n = 6). Bemcentinib significantly reduced the BLI signal in comparison with the control group (P < .0004) or to ibrutinib single treatment (P < .0247). (D) Kaplan-Meier plots showing the effect of the different treatments on the survival of mice with MCL-cell xenografts (n = 8).
Inhibition of AXL by bemcentinib reduces tumor burden and increases survival in an MCL xenograft mouse model. (A) Female nonobese diabetic severe combined immunodeficiency gamma mice were injected IV with 5 × 105 JeKo-1Luc+ cells. The mice were imaged weekly, both dorsally and ventrally (10 minutes after intraperitoneal injection with 150 mg/kg of 25 mg/mL D-luciferin). The mice were distributed in the following groups: control (vehicle only every day), bemcentinib treatment (50 mg/kg twice a day), ibrutinib treatment (25 mg/kg every day), and combination treatment (ibrutinib + bemcentinib) based on total bioluminescence. Following the assignment, there was no statistical difference between the groups (P > .99; 1-way analysis of variance test). JeKo-1Luc+ xenografts were treated for 4 weeks with vehicle every day (control), ibrutinib (25 mg/kg, every day), bemcentinib (50 mg/kg, twice a day) or the combination of ibrutinib and bemcentinib (same dose as single treatment) (n = 6). (B) Bioluminescence imaging (BLI) was assessed weekly to monitor disease progression. (C) Quantification of BLI signal from the different treatment groups in week 3 (n = 6). Bemcentinib significantly reduced the BLI signal in comparison with the control group (P < .0004) or to ibrutinib single treatment (P < .0247). (D) Kaplan-Meier plots showing the effect of the different treatments on the survival of mice with MCL-cell xenografts (n = 8).
Discussion
AXL is a tyrosine kinase receptor involved in various biological processes such as stimulation of cell proliferation and regulation of the innate immune response.42 Its role in the oncogenesis of several forms of solid cancers, including pancreatic,43 ovarian,44 and breast carcinoma,45 has been widely demonstrated. AXL expression has also been investigated in various forms of hematological malignancies. Neubauer et al34 found that the AXL transcript can be detected in several forms of leukemia, including AML38,46 and chronic lymphocytic leukemia.33 However, AXL expression has never been reported in MCL. In the present work, we demonstrate that AXL is expressed at the mRNA and protein level in MCL cell lines and patient cells. Interestingly, to the best of our knowledge, we have also demonstrated for the first time the existence of a third AXL isoform in MCL cells. To confirm the existence of the AXL3 mRNA we cloned the full transcript (Figure 1). Sequencing confirms that the exon structure of this mRNA corresponds to the predicted AXL3 isoform. Despite the isoform being predicted and described in the Ensembl database, to our knowledge, no previous reports have cloned, identified, or studied the isoform in human cells. In contrast to the 2 well-characterized AXL1 and AXL2 isoforms, the third AXL protein presents a singular extracellular structure. The transcript encodes a protein of 626 amino acids, which lacks the 2 immunoglobulin-like domains but retains the 2 fibronectin type 3–like repeats. This configuration makes this isoform a peculiar AXL receptor because there is only 1 other type of tyrosine kinase family that possesses only extracellular fibronectin type–3 domains, the reactive oxygen species tyrosine kinase family. Unlike AXL isoform 1 and 2, GAS-6 will not bind and activate the AXL3 isoform. Instead, the ligand of the fibronectin type 3–like domain remains to be formally identified. Nevertheless, some studies seem to indicate that the fibronectin type 3 domain can bind to integrin proteins,47,48 indicating that AXL activation can be triggered by cell-to-cell contact or by interaction with the extracellular microenvironment. Moreover, we cannot exclude that this third isoform can also be activated by a ligand-independent mechanism or by heterodimerization with another receptor as it was previously reported for AXL1 and AXL2.49 The AXL expression/activation pattern seems complex in MCL. We were able to detect GAS-6 transcript in most MCL cell lines (supplemental Figure 2), indicating that an autocrine activation cannot be ruled-out in MCL. These novel insights into the expression and activation of AXL isoforms may significantly impact therapeutic strategies. Pharmaceutical attempts to block AXL activation by preventing GAS-6 binding may be bypassed in cells expressing the AXL3 isoform. Splice variant switching, obtained by genetic mutation or other mechanisms, is now a well-established mechanism used by cancer cells to gain proliferation advantage or resistance to chemotherapy treatments.50-52
To further evaluate the impact of AXL inhibition in MCL cells we have used a conditional knockdown shRNA approach to decrease the expression of AXL. The shRNA construct was previously characterized to be effective in reducing AXL expression.39 Interestingly, using this approach we were able to effectively shut down AXL1 and AXL2 protein expression in the AXL shRNA JeKo-1 and AXL shRNA K562 cells. In both cell lines, AXL3 protein expression was unaffected. Despite the ability of bemcentinib to induce cell death of wild-type JeKo-1 and K562 cells, neither cell proliferation nor viability was affected by the knockdown of AXL1 and AXL2. (Figure 4). These results diverge from the reported findings of Ben-Batalla et al,38 that describe that silencing of AXL1-2 in CML cells resulted in a reduction of cell viability.38 However, the authors have not evaluated the effect of the AXL knockdown on the AXL3 protein expression that may be responsible for the observed effect on cell viability. Nevertheless, our results are in accordance with Dufies et al53 and Gioia et al,54 who have shown that AXL1-2 knockdown does not induce cell apoptosis in CML. Moreover, a study performed in the squamous cell carcinoma line (MET1) also reported that knockdown of AXL1-2 did not affect cell proliferation55 nor induce apoptosis. As shown in Figure 1, the AXL3 isoform, at the protein level, seems to be considerably more highly expressed than AXL1 and AXL2 in MCL cell lines, as well as in the CML cell line K562. The observation that AXL3 protein cannot be downregulated in our conditional shRNA system may be explained by the structure of the AXL3 mRNA. In fact, this isoform presents a long 5′ UTR sequence that may be involved in the stabilization of the mRNA. Moreover, it is also well established that mRNAs can be regulated by postsecondary modifications, like N6-adenosine methylation, that can control mRNA decay as reported for SOX2 or KLF4 mRNA.56 Therefore, it is possible to consider that different AXL mRNAs may have different lifetimes and may be more or less sensitive to a particular RNA interference sequences (shRNA or siRNA) used to induce the degradation of the AXL mRNA. To further evaluate the function of AXL3, we have custom designed a specific siRNA targeting only AXL isoform 3. As shown in Figure 4G, with this approach we were able to decrease AXL3 protein expression that was observable at 72 hours (Figure 4G). As shown in Figure 4H-I,K-M, the downregulation of AXL3 expression leads to the reduction of growth and apoptosis of MCL cells. To further characterize the function of AXL3, we took advantage of the fact that this isoform uses a different promoter than the previously described AXL isoforms, to selectively block the transcription of AXL3 by a CRISPRi approach. As illustrated in Figure 4N, induction of the sgRNA targeting the Axl3 promoter leads to a decrease of AXL3 protein expression as well as to a significant decrease in cell proliferation. Interestingly, the time course of decreased AXL3 expression was longer in the CRISPRi experiment than in the siRNA approach. However, this result is not unexpected as CRISPRi affects transcription rather than RNA degradation, and is in accordance with previous reports using CRISPRi.57,58 By using the cellular thermal shift assay, we were able to show that bemcentinib can bind the AXL3 isoform. Altogether these results indicate that the inhibition of the expression or activity of AXL3 is responsible for the growth inhibition and apoptosis of MCL cells.
Our in vitro data reveal that bemcentinib can reduce the proliferation and induces apoptosis of cells that were not killed by standard dose of ibrutinib (ie, Granta-519, Figure 3A,D). This observation is of interest because it underlines that ibrutinib resistance can be overcome by blocking AXL activation. One-third of patients treated with ibrutinib alone relapse within 2 years of treatment.26 It may therefore be valuable to investigate whether a cotreatment of MCL cells with an AXL and a next-generation BTK inhibitor, like pirtobrutinib could be favorable. To support this concept, our study also provide evidence that inhibition of AXL activity, in conjunction with the BTK inhibitor ibrutinib, increases the overall survival of MCL-carrying mice in comparison with mice treated with either ibrutinib or bemcentinib alone. Moreover, it was previously shown that cotreatment with other drugs can be beneficial to patients with MCL .59,60 In this context, the development of new therapeutic modalities simultaneously targeting several vulnerabilities identified in MCL should be of interest for the development of multimodal therapy for the long-term remission or cure of patients with MCL.
It is interesting to note that the AXL3 isoform can also be detected in other hematological (ie, chronic myelogenous leukemia) and solid (ie, pancreatic ductal adenocarcinoma) malignancies, suggesting that AXL3 may also have broader implications for the etiology of several cancer forms. In particular, the development of therapeutic antibodies targeting AXL in pancreatic or breast cancer have so far demonstrated a limited application. This may be explained, in part, by the inability of those antibodies to recognize and bind the new AXL3 isoform, permitting tumor cell evasion by this therapeutic strategy.
In conclusion, we show that MCL cells express AXL and that it contributes to the biology of MCL by promoting cell growth and survival characteristics. We also discovered that MCL cells express a third AXL isoform that is responsible for the cell growth and survival effect observed in MCL. Moreover, we also demonstrate, in a xenograft mouse model of MCL, that bemcentinib, an AXL inhibitor that received fast-track designation in non–small-cell lung cancer and is currently in clinical trials for AML, may hold promise in treating MCL as a single agent or in combination with BTK inhibitors and/or immunotherapy modalities (CAR T cells/Bi-specific T-cell engagers).
Acknowledgments
The authors thank BergenBio AS for proving the bemcentinib compound and the shRNA constructs that were used in this study, as well as Brith Bergum from the Flow Cytometry Core facility at the University of Bergen for technical advice and support. The authors also thank the Molecular Imaging Center core facility and vivarium at the University of Bergen for the help provided and Tine AS for product support.
This work was supported by research grants from the Research Council of Norway (326300 and 327278), the Norwegian Cancer Society (Kreftforeningen, 223171 and 182735), and the Western Norway Regional Health Authority (911182) (E.M.).
Authorship
Contribution: P.G., M.E.-G., and E.M. designed the experiments; P.G., M.E.-G., S.B., J.H., I.K., M.M.S, C.L., Z.F., and M.P. performed all the experimental procedures; P.G., M.E.-G., I.K., and E.M. analyzed the data; L.H., B.P., and F.B.-M. recruited patients with MCL; and P.G., M.E.-G., I.K., and E.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests and none of the authors have shares or received grants or financial support from BerGenBio AS.
Correspondence: Emmet McCormack, Department of Clinical Science, University of Bergen, Haukeland University Hospital, Jonas Lies vei 87, 5021 Bergen, Norway; e-mail: emmet.mc.cormack@med.uib.no.
References
Author notes
Sequencing data can be found in GenBank under accession numbers OR103210 (sequence 1), OR103211 (sequence 2), OR103212 (sequence 3), and OR103213 (sequence 4).
Data are available on request from the corresponding author, Emmet McCormack (emmet.mc.cormack@med.uib.no).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



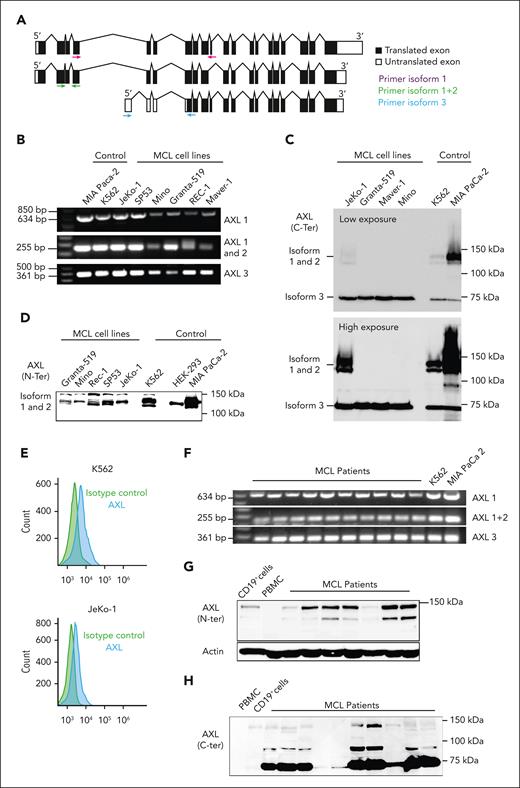
![MCL cells overexpress a new AXL isoform. (A) Schematic representation of AXL mRNA structure. The different sets of primers used for AXL mRNA amplification (reverse transcription polymerase chain reaction [RT-PCR]) are indicated in purple (specific amplification of isoform 1), green (amplification of isoform 1 and 2), and blue (specific amplification of isoform 3). The new AXL 3 isoform (bottom) is missing the first 4 coding exons of AXL isoforms 1 and 2. (B) Relative mRNA expression of the different AXL transcripts investigated by RT-PCR in MCL cell lines. All the different AXL mRNA isoforms can be detected in MCL cells. MIA-PaCa-2 and K562 were used as positive controls for AXL amplification. (C) Detection of AXL protein expression in MCL using an antibody targeting C-terminal domain of AXL. The C-terminal antibody is able to recognize all AXL isoforms. The AXL N-terminal antibody recognizes the isoform 1 and 2 but not the AXL3 isoform because the antibody epitope is not present in AXL3. K562 and MIA-PaCa-2 served as positive controls. (D) Detection of AXL protein expression in MCL using an antibody targeting the N-terminal. (E) AXL can be detected at the cell surface of MCL cells by flow cytometry staining using the AXL N-terminal antibody. K562 served as a positive control. Experiments were performed in triplicate. (F) AXL mRNA expression was investigated by RT-PCR in primary MCL cells. All the AXL isoforms were expressed in MCL primary cells. K562 and MIA-PaCa-2 served as positive controls for the AXL1 and AXL2. (G-H) AXL is expressed at the protein level in MCL cells. AXL is overexpressed in MCL cells in comparison to peripheral blood mononuclear cells (PBMCs) or CD19+ B cells. (I) AXL3 full transcript was cloned and sequenced (J-K) and corresponds to the predicted AXL3 isoform.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/17/10.1182_blood.2022015581/2/m_blood_bld-2022-015581-gr1b.jpeg?Expires=1768649258&Signature=yiadaMNNmldJx1uVgknywuNMNitCgTJwtNy5ITGg3SY8vqh1tyPsYr81mu4iOuvmXtCUbltOlzwq~RdMbohTLoT62Ach22S~3FiAMhbZXJMojCxzZNXAdrBCClEXsrj22kNAoZBPI~mFIcQv13LVgcX~z2wLXOgvZ-m3erihu0rcPrVKz4itqa5MPzb4eykdtRkITYh8ilKA4PhdYTGU2jYDj~BUr3auq3UAzJhcmeHkF674DPwBLH5PGE~A6t63SfY40mAiryKx9eNNlhE2KRRljxD9d5L8iWttZqTpxA~PVKXYKAXyK~yFfh0j3JUDY0w8qtnf~nJTeB1chsTVZw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)