Key Points
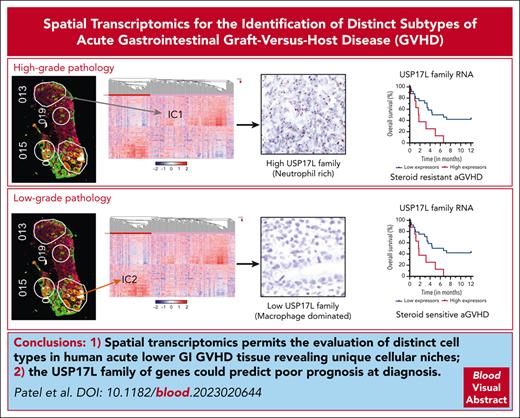
Spatial transcriptomics permits evaluation of distinct cell types in human acute lower GI GVHD tissue, revealing unique cellular niches.
Overexpression of USP17L family RNA in immune cells could predict poorer survival in lower GI aGVHD.
Abstract
Severe acute graft-versus-host disease (aGVHD) is associated with significant mortality and morbidity, especially in steroid-resistant (SR) cases. Spatial transcriptomic technology can elucidate tissue-based interactions in vivo and possibly identify predictors of treatment response. Tissue sections from 32 treatment-naïve patients with biopsy-confirmed lower gastrointestinal (GI) aGVHD were obtained. The GeoMx digital spatial profiler was used to capture transcriptome profiles of >18 000 genes from different foci of immune infiltrates, colonic epithelium, and vascular endothelium. Each tissue compartment sampled showed 2 distinct clusters that were analyzed for differential expression and spatially resolved correlation of gene signatures. Classic cell-mediated immunity signatures, normal differentiated epithelial cells, and inflamed vasculature dominated foci sampled from steroid-sensitive cases. In contrast, a neutrophil predominant noncanonical inflammation with regenerative epithelial cells and some indication of angiogenic endothelial response was overrepresented in areas from SR cases. Evaluation of potential prognostic biomarkers identified ubiquitin specific peptidase 17–like (USP17L) family of genes as being differentially expressed in immune cells from patients with worsened survival. In summary, we demonstrate distinct tissue niches with unique gene expression signatures within lower GI tissue from patients with aGVHD and provide evidence of a potential prognostic biomarker.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative treatment for patients with high-risk malignant and nonmalignant hematologic conditions. The occurrence of severe acute graft-versus-host disease (aGVHD), particularly of the lower gastrointestinal (GI) tract, is a leading cause of early nonrelapse mortality (NRM) after HCT.1 Recent investigation has elucidated detailed biological mechanisms that drive aGVHD involving interactions between donor T cells, injured host tissue, intestinal microbial species, and numerous immune cell subsets.2,3 These advances have led to the application of novel therapeutic agents, many of which are currently under investigation.4
A major challenge remains how to best identify which patients with aGVHD will not be responsive to standard corticosteroid therapy, as this risk stratification is of high clinical importance and key to optimal trial design for novel agents. To date, serum biomarkers have emerged as an important tool in predicting clinical outcomes after HCT. Studies have previously demonstrated that the measurement of 2 plasma biomarkers, suppressor of tumorigenicity-2 (ST2) and regenerating islet-derived 3α (REG3α), at the diagnosis and during the treatment of aGVHD can predict NRM.5-7 However, despite these advances in blood-based biomarkers of aGVHD, comprehensive tissue-based evaluation of aGVHD are currently lacking. Tissue-based examination has the potential to provide a more accurate assessment of aGVHD-related immune signatures, particularly if the immune cells are restricted to the affected organs. The ability to visualize aGVHD at the tissue level provides unique information on the cell types affected by the disease and the spatial relationship of immune cells with other cell types in the microenvironment. This provides mechanistic insight into the key local cell-cell interactions that drives different phenotypes of aGVHD and opens the door to discovering new diagnostic biomarkers and novel therapeutic avenues.
We hypothesized that gene expression profiles in epithelial, endothelial, and immune cells in the GI tract would identify distinct types of aGVHD that are associated with prognostic differences in outcomes. To test this hypothesis, we performed spatial transcriptomics on tissue sections obtained from treatment-naïve patients with biopsy-confirmed aGVHD of the lower GI tract. Two clear major areas of interest (AOIs) clusters were identified for epithelial, immune, and endothelial compartments. A classic cell-mediated immunity (CMI) dominated inflammatory immune signature–dominated AOIs sampled from steroid-sensitive (SS) aGVHD cases. Contrarily, a noncanonical, neutrophil-dominated inflammatory signature with evidence of DNA repair ubiquitin specific peptidase 17–like (USP17L) family of genes as potential prognostic biomarkers, given their differential expression in immune cells from patients with worsened survival. Our findings characterize unique gene expression patterns in distinct tissue niches of lower GI aGVHD and suggest further investigation of a novel tissue-based prognostic biomarker.
Materials and methods
Human biospecimen procurement
The current study was approved by the Institutional Review Board at the Massachusetts General Hospital (protocol 2013P001854). We identified 32 patients who were diagnosed and treated for aGVHD of the lower GI tract at Massachusetts General Hospital between 2016 and 2021 and had archived, paraffin-embedded tissue from the colonic biopsy at the time of GVHD diagnosis available for evaluation. All biopsy tissue was obtained before the initiation of systemic treatment. Patients had no evidence of concurrent GI infection at the diagnosis of aGVHD. Clinical data were extracted from the institutional database and individual medical record of each patient. Patients were classified as having either SS or SR aGVHD to associate gene expression signatures with clinical outcomes. Day 28 from initiation of GI aGVHD systemic treatment was chosen on the basis of data on end points in patients with aGVHD.8 Patients were classified as having SS aGVHD if (1) symptoms improved with first-line treatment and (2) the patient remained alive and without the initiation of second-line treatment by day 28. Patients were classified as having SR aGVHD if (1) symptoms did not improve or progressed with first-line treatment, (2) the patient received second-line treatment, or (3) the patient died before day 28.
Spatial transcriptomics data generation
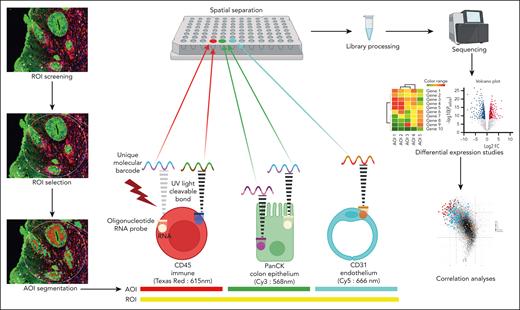
Archived, paraffin-embedded endoscopic colon biopsies from 32 patients comprised the key material for the study. All the biopsies were obtained at diagnosis from treatment-naïve cases. On serial sections made from these blocks, the first was stained for hematoxylin and eosin, and the morphology was reviewed by 2 pathologists (V.D. and B.K.P.). A modified Lerner grading system was used to classify various foci as low-grade (LG) pathology (grade 1, apoptosis only; and grade 2, single crypt loss) and high-grade (HG) pathology (grade 3, contiguous crypt loss; and grade 4, extensive ulceration/denudation).9,10 The second section was used to collect genetic information from transcribed mRNA on GeoMx Digital Spatial Profiler platform using the Human Whole Transcriptome Atlas of 18 676 RNAs (NanoString Technologies, Seattle, WA) and fluorescent morphology markers (PanCK and CD45 [ready-to-use proprietary NanoString probes]) and CD31 (ab76533; dilution 1:40; Abcam) conjugated with lyophilized Alexa Fluor 647 mix ab274049 (Abcam) to visualize the epithelial, immune, and endothelial compartments, respectively, at different emission spectra.11 Once stained, guided by hematoxylin and eosin morphology, ≈300-μm diameter-sized regions of interest (ROIs) were marked, and “segmentation” of the ROIs into distinct immune/epithelial/endothelial “areas” of interest (AOIs) was checked before finalizing the collection of the barcodes (attached to oligos by ultraviolet light cleavable bonds) onto a 96-well collection plate. The collection was sequential and separate for every AOI, ultimately achieving a spatial transcriptomic map (Figure 1). Barcoded oligos were then prepared for Illumina-based sequencing to generate target probe-specific count files and saved in digital count conversion format (see supplemental Methods, available on the Blood website).
Spatial transcriptomics workflow on the GeoMx Digital Spatial Profiler. Within a delineated ROI, guided sampling of molecular barcodes from the unique fluorescence-segmented AOIs occurs within the instrument. These barcodes are bound to probes against nearly 18 676 RNAs by ultraviolet light-sensitive bonds. Sampling is nonoverlapping and sequential, ensuring spatial separation of probes from each AOI. A library is prepared from the extracted probes and sequenced, and raw data files are generated with information on number of RNA transcripts per gene for every AOI. Differential expression analysis and correlation studies follow, providing leads on disease subtypes and tissue neighborhood interactions. PanCK, pan cytokeratin.
Spatial transcriptomics workflow on the GeoMx Digital Spatial Profiler. Within a delineated ROI, guided sampling of molecular barcodes from the unique fluorescence-segmented AOIs occurs within the instrument. These barcodes are bound to probes against nearly 18 676 RNAs by ultraviolet light-sensitive bonds. Sampling is nonoverlapping and sequential, ensuring spatial separation of probes from each AOI. A library is prepared from the extracted probes and sequenced, and raw data files are generated with information on number of RNA transcripts per gene for every AOI. Differential expression analysis and correlation studies follow, providing leads on disease subtypes and tissue neighborhood interactions. PanCK, pan cytokeratin.
Computational analysis
The RNA data were quantile normalized, their background expression was estimated, and unsupervised hierarchical clustering was performed on the top 500 most variable genes. Subsequently, the AOIs exhibiting most differential expression (DE) were selected for individual component heat mapping by unweighted pair group method with arithmetic mean. Clear distinction (first level of dendrogram separation) between the AOIs (columns) for gene expression (rows) from the 3 tissue compartments was identified on any heat map as a first step. Visually, there were at least 2 clear clusters vertically (for AOIs) and ≥2 clusters horizontally (for differentially expressed RNA) in each histologic compartment. These vertical clusters were named per their tissue compartment suffixed with 1 and 2, example immune clusters 1 and 2 (IC1 and IC2, respectively) and similarly EC1 and EC2 for epithelial clusters and VC1 and VC2 for vascular endothelial clusters. Between these AOI-based clusters, the Fisher exact test was used to compare proportions of AOIs from patients representing a particular clinical parameter. Volcano plots were constructed to visualize the most DE genes between groups.
The horizontal immune clusters and the differentially overexpressed genes were subjected to gene set enrichment analysis using the Molecular Signatures Database site (https://www.gsea-msigdb.org) with the curated hallmark and Gene Ontology data sets at an upper false discovery rate/q value of 0.05. As an alternative, the STRING database (https://string-db.org) was used to look for likely additional protein-protein interactions at a significance level of 0.07 (ie, high confidence). For hub network analysis (most highly interacting set of proteins), cytoHubba application on Cytoscape (https://cytoscape.org) was used to identify the top 10 most interactive genes. SpatialDecon (https://www.bioconductor.org/packages/release/bioc/html/SpatialDecon.html) was used to perform immune cellular deconvolution to identify relative cellular composition of immune clusters.
Supervised differential expression analysis was performed comparing gene expression from patients with aGVHD with early mortality, defined as NRM mortality within 6 months from diagnosis, with the other aGVHD cases to ascertain genes with predictive potential. Relevant survival and mortality parameters were performed with Kaplan-Meier analysis, and their significance was determined on Mantel-Cox log-rank test.
Correlation analysis
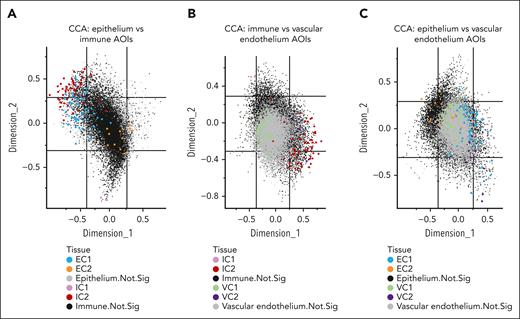
To systematically understand how genes correlated across immune and epithelial compartments, we performed canonical correlation analyses and visualized the representative DE genes of the 6 IC, EC, and VC gene signature–based clusters on a correlation circle plot.12 A dimensionality reduction technique, canonical correlation analysis aims to project 2 high-dimensional data sets (eg, immune and epithelial count matrices, where rows represent gene expression in AOIs and columns represent genes) measured on the same paired samples (the rows are ordered such that matching rows in the 2 counts matrices correspond to same ROIs) onto a lower dimensional space to visualize groups of genes that have similar trends across samples. The axes (termed canonical components or canonical covariates) in each plot were constructed to maximize the correlation between genes counts in paired samples. On each plot, each dot represents a gene. Each gene’s coordinate is determined by its correlation with the first (x-axis) and second (y-axis) canonical components. The individual coordinates and the angle between them are both important to estimate the correlation between any pair of genes in a correlation circle plot. With the genes positioned as imaginary vectors from the plot’s center, an acute angle between 2 gene vectors would indicate a positive correlation. Conversely, an obtuse angle would indicate a negative correlation, and a right angle would imply no correlation.
RNA in situ hybridization assessment
Automated chromogenic RNA in situ hybridization (RNA-ISH) assay was performed using the Advanced Cell Diagnostics RNAscope 2.5 LS Reagent Kit - BROWN (catalog number 322100) on the BondRx 7.0 platform (Leica Biosystems Inc, Buffalo Grove, IL). Assay was performed using a custom probe from Advanced Cell Diagnostics against USP17L (RNAscope 2.5 LS Probe-Hs-USP17L-O1-C1; probe design number NPR-0039096) used at a 1:100 dilution and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ready-to-use RNAscope 2.5 LS Probe-Hs-GAPDH; catalog number 310328). Quantification of RNA-ISH was performed on Halo AI imaging platform, version 3.6.4134 (Indica Labs, Albuquerque, NM) using the ISH-immunohistochemistry (IHC) version 3.1.4 module to identify USP17L RNA-positive immune cells in the stroma. IHC with CD66b (Abcam, Cambridge, United Kingdom; clone EPR25354-2; ab 300122) for granulocytes involved heat-mediated antigen retrieval with Tris-EDTA buffer (pH 9.0; epitope retrieval solution 2) for 20 minutes followed by a 30-minute room temperature incubation in 1:20 000 dilution of antibody on a Leica Biosystems BOND RX 7.0 instrument. This was followed by a ready-to-use LeicaDS9800 (BOND Polymer Refine Detection) application.
Results
Patient characteristics
Patient, transplant, and GVHD characteristics are provided in Table 1. The median age at transplant was 62 years (range, 23-77 years), and 19 (59%) of the patients were men. The most common indication for transplant was acute myeloid leukemia. Transplantation was performed predominantly from human leukocyte antigen–matched donors (n = 24 [75%]), using reduced-intensity conditioning regimens (n = 22 [69%]), and with peripheral blood stem cell grafts (n = 30 [94%]). The most common GVHD prophylaxis regimen was the combination of tacrolimus and methotrexate (n = 20 [63%]). The median time from transplant to lower intestinal aGVHD diagnosis was 49 days (range, 20-152 days). At day 28 after initiation of systemic therapy with corticosteroids, 13 patients (41%) were classified as SS and 19 patients (58%) were classified as SR. Median follow-up of survivors was 4 months (range, 4 days to 20 months). During the follow-up period, there were 23 deaths. The most common primary cause of death was aGVHD (n = 12 [52%]).
Spatial transcriptomics identifies distinct immune cell niches
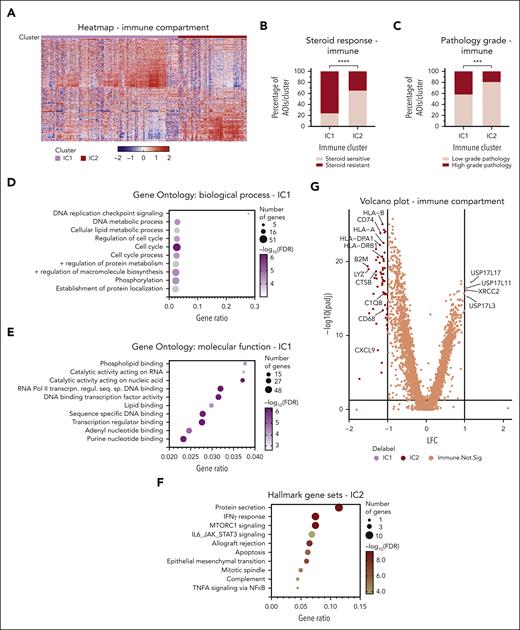
Thirty-two aGVHD tissue specimens were processed for spatial transcriptomics analysis of 309 ROIs comprising a total of 720 AOIs (Figure 1) sampled from epithelial, endothelial, and immune areas. Immune cells were represented in 94% of the ROIs, making them the most sampled subset, with information from 57 821 cells over 291 AOIs. Epithelial cells were sampled in 78.6% of ROIs (243 AOIs with 33 501 cells). Endothelial transcriptomes were the least abundant, with only 2298 cells sampled from 186 AOIs (supplemental Table 1). Focusing on the immune cells, unsupervised hierarchical clustering demonstrated 2 clear clusters, IC1 and IC2 (Figure 2A). The IC1 cluster had an overrepresentation of AOIs derived from patients with SR GVHD (Fisher exact P < .0001) and HG pathology areas (Fisher exact P = .0007) (Figure 2B-C). When individual patient(s) were assigned to IC1 or IC2 category based on majority of AOIs (>50%) being either, the statistical significance for steroid responsiveness was lost while comparing ranks using Mann-Whitney rank-sum test (data not shown). This highlights the significant intrapatient heterogeneity of regional immune responses that underlies aGVHD. We performed gene set enrichment analysis to characterize IC1 and IC2 clusters. IC1 was enriched for genes involved with cell cycle and transcription-relevant DNA binding. The IC2 cluster, on the other hand, showed increased transcription of classic CMI (and thereby its prototype, allograft rejection) pathway mediator genes, such as those in the hallmark gene sets of downstream tumor necrosis factor-α and interferon gamma signaling (Figure 2D-F). Differential expression analysis between IC1 and IC2 AOIs identified genes that were driving separation of these immune response (Figure 2G and supplemental Table 2). IC2 had significantly higher expression of antigen-presentation machinery (human leukocyte antigen and associated molecule families) and inflammatory mediators (complement, lysozyme, and chemokines), indicating a classic CMI cascade. The IC1 cluster did not have a clear set of genes enriched, but there was high expression of USP17L-family member genes. XRCC2, a DNA damage repair gene with no specific known immune function, was the only other gene with at least a 2-fold higher expression in the IC1 cluster.
Immune compartment transcriptional analysis in aGVHD of colon. (A) Two clusters visualized in this unsupervised heat map, IC1 and IC2. Rows represent genes, and each column is an AOI. (B-C) A preponderance of AOIs from LG pathology areas and SS patients in IC2. P value calculated by Fisher exact test. ∗∗∗P = .0007; ∗∗∗∗P < .0001. (D) Hallmark gene set enrichment for IC2 genes with interferon gamma (IFN-γ) response. (E-F) Gene Ontology enrichment for the genes upregulated in IC1 shows involvement of multifaceted DNA-binding pathways principally directed toward cell cycle regulation. (G) Volcano plot of differentially expressed genes between IC1 and IC2. FDR, false discovery rate; RNA Pol II transcrpn. regul. seq. sp. DNA binding, RNA polymerase II transcription regulatory region sequence-specific DNA binding.
Immune compartment transcriptional analysis in aGVHD of colon. (A) Two clusters visualized in this unsupervised heat map, IC1 and IC2. Rows represent genes, and each column is an AOI. (B-C) A preponderance of AOIs from LG pathology areas and SS patients in IC2. P value calculated by Fisher exact test. ∗∗∗P = .0007; ∗∗∗∗P < .0001. (D) Hallmark gene set enrichment for IC2 genes with interferon gamma (IFN-γ) response. (E-F) Gene Ontology enrichment for the genes upregulated in IC1 shows involvement of multifaceted DNA-binding pathways principally directed toward cell cycle regulation. (G) Volcano plot of differentially expressed genes between IC1 and IC2. FDR, false discovery rate; RNA Pol II transcrpn. regul. seq. sp. DNA binding, RNA polymerase II transcription regulatory region sequence-specific DNA binding.
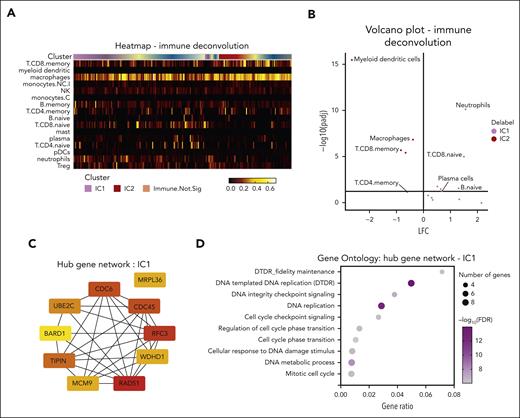
To better understand the immune cell types involved with these gene expression differences, we used SpatialDecon, a cellular deconvolution method of the immune clusters. This revealed significant enrichment of key antigen-presenting cells (APCs), including dendritic cells and macrophages, along with the terminal memory CD8 T cells in IC2, which is consistent with the classic CMI response (Figure 3A-B). In contrast, IC1 had a predominantly neutrophil infiltrate with some evidence of plasma cell and naïve (unstimulated) CD8 T cells. Together with the absence of APCs, this points to a form of impaired CMI associated with a noncanonical, neutrophil (innate) and potential plasma cell (humoral) immune response in the IC1 cluster with linked HG pathology and SR disease. Additional protein interactome analysis of IC1-enriched genes using the STRING database and hub network identified genes such as RAD51, RFC3, and TIPIN, among others, related to DNA replication and repair (Figure 3C-D).
Characterization of IC1 aGVHD noncanonical immune niche. (A) The immune deconvolution heat map, showing distribution of different cellular phenotypes across clusters. (B) Volcano plot of differential enrichment of immune cell types. (C) Hub gene analysis of the top 10 most interacting genes/proteins enriched in IC1, identified by CytoHubba application on Cytoscape software. Red to yellow coloring correlated with decreasing interaction stores. (D) Gene Ontology pathways enriched in IC1, demonstrating multiple gene sets associated with DNA damage and cell cycle.
Characterization of IC1 aGVHD noncanonical immune niche. (A) The immune deconvolution heat map, showing distribution of different cellular phenotypes across clusters. (B) Volcano plot of differential enrichment of immune cell types. (C) Hub gene analysis of the top 10 most interacting genes/proteins enriched in IC1, identified by CytoHubba application on Cytoscape software. Red to yellow coloring correlated with decreasing interaction stores. (D) Gene Ontology pathways enriched in IC1, demonstrating multiple gene sets associated with DNA damage and cell cycle.
Further analysis of pooled USP17L gene transcripts with the cellular deconvolution algorithm showed their expression to be most correlated with neutrophil percentage (Pearson correlation coefficient, r = 0.5544) and anticorrelated with macrophages (Pearson r = −0.3086) (supplemental Figure 1A-D). This agrees with Figure 3B, indicative of a defining role of the USP17L genes in the IC1 niche. The presence of increased neutrophils in high USP17L cases was confirmed with IHC for CD66b (supplemental Figure 1E).
Epithelial and vascular endothelial cell regions separate based on inflammatory response
Epithelial cell AOI analysis by unsupervised hierarchical clustering noted 2 clear clusters (EC1 and EC2) identified in our aGVHD samples (Figure 4A and supplemental Table 2). DE analysis of genes between epithelial clusters (Figure 4B) identified EC1 as a normal differentiated colon state and EC2 as an inflamed state with indication of regenerative stem cell features. EC1 had higher expression of colon-specific keratin 20 (KRT20) and fatty acid–binding protein 1 (FABP1), which are expressed in normal proliferating colon epithelium,13 and guanylin (GUCA2A), a known amino acid peptide secreted by normal goblet cells in the colon.14 EC2, on the other hand, was rich in genes that transcribe chemokines, key antimicrobial peptides (REG family members, including REG3A), and genes that influence stem cell function, such as CLU, SINHCAF, and L1TD1, indicative of a regenerative epithelial response. Notably, the normal differentiated EC1 arm had significant association with LG pathology (Fisher exact P = .0186), and EC2 with steroid resistance (Fisher exact P < .001) (Figure 4C-D). EC2 foci were also overrepresented in patients who received reduced-intensity conditioning (Fisher exact P = .0154); no association with conditioning intensity was observed in the other cell compartments. The vascular endothelial cells also demonstrated 2 distinct groups with unsupervised hierarchical clustering (Figure 4E and supplemental Table 2). The VC2 cluster showed gene expression of inflammatory response (IL32) and extracellular matrix (COL6A2, TIMP1, and TNC) genes (Figure 4F). Although there were several genes enriched in VC1 (Figure 4D), a consistent theme with known gene sets was lacking. Instead, there was expression of some proangiogenic factors (WNT10A and SCUBE2). Also, VC1 had significantly more AOIs from LG pathology foci (Fisher exact P = .035). These clusters were not different between SS and SR patients (Fisher exact P = .6032) (Figure 4G-H).
Transcriptomic profiling of colonic epithelium and vascular endothelium compartments in aGVHD of colon. (A) Unsupervised heat map of epithelial compartment, with the 2 most different hierarchical clusters marked. Rows represent genes, and each column is an AOI. (B) Epithelial volcano plot shows differentially expressed genes pertaining to mature colon epithelium with basal function and with minimal inflammatory presence in EC1 and an obvious inflammatory, chemotactic signature with some evidence of stem cell genes (regenerative activity?) in EC2. (C) The inflamed EC2 niche is enriched in SR patients (Fisher exact test, ∗∗∗∗P < .0001). (D) The basal EC1 niche has more tissue with LG damage (Fisher exact test, ∗P < .0186). (E) Unsupervised heat map of endothelial compartment similarly shows 2 clusters with (F) vascular cluster 2 (VC2) differentially expressing inflammatory genes. VC1 is a basal-leaning signature with genes related to solute channels, lipid handling, and cell-matrix interactions, and some genes pertaining to neoangiogenesis. (G) Neither cluster is overrepresented in differing steroid response (Fisher exact test, P = .6032), but (H) the inflamed, VC2 vessels are increased in HG pathology areas (Fisher exact test, ∗P = .0350).
Transcriptomic profiling of colonic epithelium and vascular endothelium compartments in aGVHD of colon. (A) Unsupervised heat map of epithelial compartment, with the 2 most different hierarchical clusters marked. Rows represent genes, and each column is an AOI. (B) Epithelial volcano plot shows differentially expressed genes pertaining to mature colon epithelium with basal function and with minimal inflammatory presence in EC1 and an obvious inflammatory, chemotactic signature with some evidence of stem cell genes (regenerative activity?) in EC2. (C) The inflamed EC2 niche is enriched in SR patients (Fisher exact test, ∗∗∗∗P < .0001). (D) The basal EC1 niche has more tissue with LG damage (Fisher exact test, ∗P < .0186). (E) Unsupervised heat map of endothelial compartment similarly shows 2 clusters with (F) vascular cluster 2 (VC2) differentially expressing inflammatory genes. VC1 is a basal-leaning signature with genes related to solute channels, lipid handling, and cell-matrix interactions, and some genes pertaining to neoangiogenesis. (G) Neither cluster is overrepresented in differing steroid response (Fisher exact test, P = .6032), but (H) the inflamed, VC2 vessels are increased in HG pathology areas (Fisher exact test, ∗P = .0350).
Spatial correlation analysis demonstrates differences in cellular microenvironment niches
To understand the relationship between different cell types in aGVHD response, gene signatures of immune, epithelial, and vascular cells were correlated with each other to define cellular microenvironment niches (Figure 5). Gene signatures from classic cell-mediated immune response (IC2) and normal differentiated epithelium (EC1) strongly coclustered spatially on tissue using this unbiased correlation approach (Figure 5A). In addition, IC2 was correlated with inflamed vascular endothelium VC2 cell groups (Figure 5B). In contrast, the relationship of epithelial and vascular cell compartments appeared to be similar between the different clusters, although there was some tendency of EC1-VC2 and EC2-VC1 cocorrelation (Figure 5C). Thus, these findings support the belief that the regional immune response drives the integrated cellular microenvironment response in this cohort of aGVHD specimens. Altogether, the dominant IC2-VC2-EC1 cellular niche reflects the classic cellular-mediated immune response that is generally SS, whereas a combination of other cell type clusters represents a collection of heterogeneous SR pathology.
Spatial transcriptional correlation analysis between immune, epithelial, and endothelial compartments in aGVHD of colon. Canonical correlation analyses (CCAs) were performed to visualize genes that correlate together across the different compartments of (A) epithelium and immune AOIs; (B) immune and vascular endothelium AOIs; and (C) epithelium and vascular endothelium AOIs. The axes (termed canonical components or canonical covariates) were constructed to maximize the correlation between gene counts in immune and epithelial AOIs in the same ROI. On this CCA plot, each dot represents a gene. Each gene’s coordinate is determined by its correlation with the first (x-axis) and second (y-axis) canonical components. The correlation between 2 genes is positive if the angle between them is sharp (acute) and negative if the angle is obtuse.
Spatial transcriptional correlation analysis between immune, epithelial, and endothelial compartments in aGVHD of colon. Canonical correlation analyses (CCAs) were performed to visualize genes that correlate together across the different compartments of (A) epithelium and immune AOIs; (B) immune and vascular endothelium AOIs; and (C) epithelium and vascular endothelium AOIs. The axes (termed canonical components or canonical covariates) were constructed to maximize the correlation between gene counts in immune and epithelial AOIs in the same ROI. On this CCA plot, each dot represents a gene. Each gene’s coordinate is determined by its correlation with the first (x-axis) and second (y-axis) canonical components. The correlation between 2 genes is positive if the angle between them is sharp (acute) and negative if the angle is obtuse.
Identification of prognostic biomarkers for aGVHD
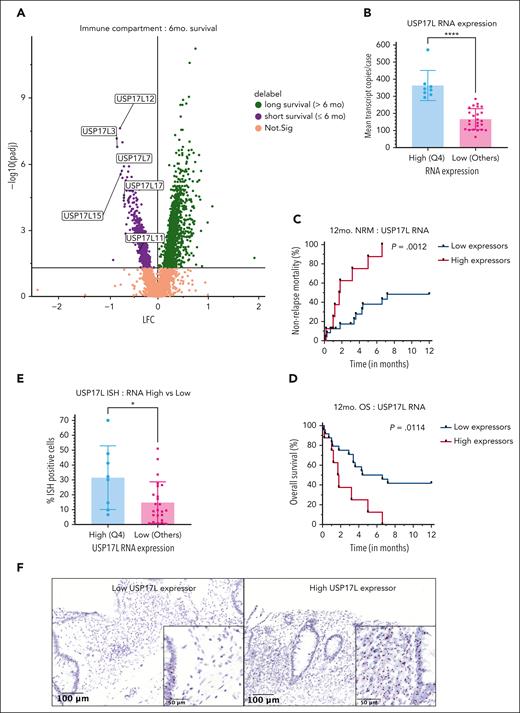
We explored the possibility of predicting poorer outcomes at diagnosis, by examining differences in gene expression in patients with varied survival, irrespective of their future steroid response (Figure 6A). Notably, multiple USP17L family members (USP17L3, USP17L7, USP17L11, USP17L12, USP17L15, and USP17L17) were among the most significant genes enriched in patients with nonrelapse deaths within 6 months of lower GI aGVHD diagnosis (Figure 6A). Some of these genes were also overrepresented in the IC1 niche from unsupervised analysis (Figure 2G).
Biomarkers with relevance to survival in SS colon aGVHD. (A) Members of USP17L gene family are overexpressed in immune cells of patients with aGVHD with NRM within 6 months. (B) Quartile-based assessment of combined USP17L (USP17L3, USP17L7, USP17L11, USP17L12, USP17L15, and USP17L17) RNA, used to define “high expressors” as those cases with mean pooled USP17L transcripts in fourth quartile (Q4) showed significant difference with the other quartiles (pooled as “low expressors”) (unpaired t-test, ∗∗∗∗P < .0001). Kaplan-Meier curve comparing combined USP17L high vs low expressors for (C) NRM (log-rank P = .0012) and (D) overall survival (log-rank P = .0114) showed better survival in low expressors. (E) RNA-ISH staining for USP17L family on the cases shows significant stronger staining in the USP17L RNA transcript high expressor cohort (unpaired t-test, ∗P = .0156). (F) USP17L isoform RNA-ISH staining validation on paraffin-embedded archival tissue in a low (left panel) and a high (right panel) expressor case (×17 magnification with scale bar = 100 μm). Inset of low expressor panel shows staining of USP17L RNA (brown-black granules) predominantly in glandular epithelium (left edge) and minimally on immune cells. Inset of high expressor panel shows comparatively stronger staining of immune population (left half) as well as in the epithelium (inset magnification ×60 with scale bar = 50 μm). OS, overall survival.
Biomarkers with relevance to survival in SS colon aGVHD. (A) Members of USP17L gene family are overexpressed in immune cells of patients with aGVHD with NRM within 6 months. (B) Quartile-based assessment of combined USP17L (USP17L3, USP17L7, USP17L11, USP17L12, USP17L15, and USP17L17) RNA, used to define “high expressors” as those cases with mean pooled USP17L transcripts in fourth quartile (Q4) showed significant difference with the other quartiles (pooled as “low expressors”) (unpaired t-test, ∗∗∗∗P < .0001). Kaplan-Meier curve comparing combined USP17L high vs low expressors for (C) NRM (log-rank P = .0012) and (D) overall survival (log-rank P = .0114) showed better survival in low expressors. (E) RNA-ISH staining for USP17L family on the cases shows significant stronger staining in the USP17L RNA transcript high expressor cohort (unpaired t-test, ∗P = .0156). (F) USP17L isoform RNA-ISH staining validation on paraffin-embedded archival tissue in a low (left panel) and a high (right panel) expressor case (×17 magnification with scale bar = 100 μm). Inset of low expressor panel shows staining of USP17L RNA (brown-black granules) predominantly in glandular epithelium (left edge) and minimally on immune cells. Inset of high expressor panel shows comparatively stronger staining of immune population (left half) as well as in the epithelium (inset magnification ×60 with scale bar = 50 μm). OS, overall survival.
To further evaluate the prognostic relevance of the USP17L family of genes, we undertook a quartile-based assessment. The median expression of the combined 6 USP17L genes (normalized for cell count and area) for the 32 cases was 198.9 units per immune AOI sampled, with an interquartile range of 161.8 units per immune AOI. We compared survival between highest quartile (quartile 4), assigned as “high expressors,” with the rest of the cohort (quartiles 1-3), being labeled as “low expressors” (supplemental Table 3). This resulted in classification of 80 “high” and 169 “low” expressor immune AOIs. As expected, the level of combined USP17L family RNA expression was significantly different between the high and low transcript expressors (unpaired t-test: P < .0001) (Figure 6B). Analysis of all aGVHD specimens revealed significantly shorter survival in these high expressors (NRM: log-rank P = .0012; overall survival: log-rank P = .0114) (Figure 6C-D).
We validated USP17L isoform expression with RNA-ISH in our aGVHD cohort, confirming stronger positivity in immune cells from these high expressors using quantitative Halo AI digital imaging software (unpaired t-test: P = .0156) (Figure 6E-F). However, there were some discordant results between the assays, with an associated moderate positive correlation between USP17L family RNA expression by NanoString GeoMx (Pearson coefficient, r = 0.6504) (supplemental Figure 2A). Although multiregion analysis with NanoString GeoMx identified the combined USP17L genes as prognostic, the quantitative USP17L isoform RNA-ISH assay separating strong and weak positivity was not prognostic in this cohort of samples (supplemental Figure 2B-D). RNA-ISH with GAPDH as a control for RNA quality showed heterogeneous staining in most biopsies with discrepant RNA expression and ISH positivity, indicating potential tissue processing issues contributed to the differences in these assays (supplemental Figure 2E).
Discussion
Despite recent therapeutic advances, steroid-refractory aGVHD of the lower intestinal tract remains a life-threatening complication of allogeneic HCT. Novel approaches to identify patients who are destined to develop steroid-refractory disease are important in clinical practice and would be extremely helpful in designing risk-stratified approaches to treatment for this biologically heterogeneous disease. In the current study, spatial transcriptomics were used to evaluate tissue sections of treatment-naïve lower GI aGVHD. Foci of immune infiltrates, colonic epithelium, and vascular endothelium showed 2 distinct gene expression clusters in each tissue compartment. Their impact on each other within spatial neighborhood niches was evaluated with canonical correlation analysis. The findings are summarized as a schema in Figure 7. Classic CMI immune signatures, normal differentiated epithelial cells, and inflamed vasculature dominated foci sampled from SS cases. In contrast, neutrophil-predominant noncanonical inflammation with regenerative epithelial cells and some indication of angiogenic endothelial response was overrepresented in areas from SR cases. We also identified the USP17L family of genes as potential prognostic tissue-based biomarkers, as they were highly DE in immune cells from patients with aGVHD with worsened survival, irrespective of their prospective steroid response. USPL17L isoform expression was validated with RNA-ISH, providing a potential path toward clinical implementation of this potential biomarker for severe aGVHD. However, differences between the NanoString and RNA-ISH platforms require further refinements to fully develop a clinical assay to validate in a larger cohort of tissue samples.
Proposed schema of pathophysiology in aGVHD of colon in the context of steroid response. A typical SS focus would have immune cells related to classic CMI response, amenable to steroid (S) therapy. The epithelium would be leaky with overactive ion channels, having experienced LG damage with mainly apoptosis and the occasional crypt loss. The endothelium would show an inflammatory signature. Contrarily, an SR focus would have a neutrophil-rich immune milieu with stunted CMI response, possibly secondary to genotoxic damage to host-derived antigen-presenting cells. There would be HG pathology in colonic epithelium with extensive crypt loss, and the vessels would exhibit a near basal functioning phenotype with some neoangiogenic signatures.
Proposed schema of pathophysiology in aGVHD of colon in the context of steroid response. A typical SS focus would have immune cells related to classic CMI response, amenable to steroid (S) therapy. The epithelium would be leaky with overactive ion channels, having experienced LG damage with mainly apoptosis and the occasional crypt loss. The endothelium would show an inflammatory signature. Contrarily, an SR focus would have a neutrophil-rich immune milieu with stunted CMI response, possibly secondary to genotoxic damage to host-derived antigen-presenting cells. There would be HG pathology in colonic epithelium with extensive crypt loss, and the vessels would exhibit a near basal functioning phenotype with some neoangiogenic signatures.
Serum biomarkers are now well established in the setting of aGVHD. The Mount Sinai Acute GVHD Consortium (MAGIC) algorithm probability, generated from measurements of ST2 and REG3α, successfully predicts NRM in patients with aGVHD better than clinical response.5,6 The use of the MAGIC algorithm probability is already being used in risk-adapted approaches to the prevention or treatment of aGVHD in prospective trials.15-17 Currently, there is a distinct paucity of tissue-based biomarkers, yet most patients with lower GI aGVHD will have diagnostic tissue sampled. Furthermore, a tissue-based biomarker could have the potential to be combined with blood-based biomarkers and achieve a real-time snapshot of aGVHD biology and enhance risk stratification.
The most pronounced gene expression signature was identified within the immune infiltrate. Although classic CMI pathways were predominant in SS patients, most AOIs from SR patients had neutrophil-predominant noncanonical inflammation. These findings are in line with previous findings that neutrophil abundance (>20 per field) in lower intestinal GVHD tissue is associated with increased early NRM.18 Derived from the immune deconvolution algorithm, the IC1 foci had a near 3-fold increase (9.1% vs 3.2%) in neutrophils over the IC2 foci (data not shown). Thus, it appears steroid resistance is intrinsic to aberrant immune response in these microscopic pockets of HG pathology. At a gene level, the hub network of most interacting proteins has a strong DNA damage-repair pathway activity in the SR AOIs. Coupled with the relative lack of classic CMI signatures in the SR arm, we hypothesize that damage to tissue-resident APCs is a root cause for these findings.19 The USP17L family of genes was chosen for RNA-ISH validation from the IC1 arm given the multiple significantly elevated paralogs and the potential to use collective gene expression for robust signal generation in archival tissue to enable better ease of interpretation. Genes of this family are deubiquitinases that salvage proteins from proteasomal degradation and have diverse functions, including promoting DNA repair.20 In the context of immunity, a key function of these genes is as master regulators of T helper 17 cells through activation of retinoic orphan receptor-γ (RORC) gene.21 We, however, found no such evidence of T helper 17 cell enrichment in the IC1 arm.
Our findings agree with an RNA-sequencing based transcriptome assessment of fatal intestinal SR aGVHD.22 Unlike our study, the investigators evaluated GVHD biopsies at 2 time points: at diagnosis and following steroid nonresponse. Concordant with their study, analysis of our EC2 AOIs demonstrated an overexpression of cytokines (CC18 and CXCL9) and a diverse array of genes (CHI3L1, SETD7, NNMT, TGM2, PDPN, and IGFBP5) along with a paucity of CA1 and SELENBP1 (overexpressed in EC1) (supplemental Table 2). Contrary to their observation, PLA2G2A was underexpressed in their normal colon biopsies, but was enriched in our EC1, which represented the basal colonic state. The investigators also identified higher expression of genes associated with DNA damage (GK, PGK1P2, HIST1H1A, and COX7BP2) among patients with early death, albeit different genes than those observed in our study (ie, USP17L).
This current study has multiple limitations, aside from the limited number of patient samples and retrospective nature of the analysis. First, spatial transcriptomics is used as a discovery tool; thus, the findings require external validation. Second, the lack of a statistically significant extrapolation of AOI-based inferences to a whole patient is a detriment. Third, the relative contribution to pathology between the donor and host immune cell populations could not be elaborated given the construct of an ROI. Emerging single-cell tissue transcriptomic tools could shed light into these limitations of the current study. Finally, the RNA-ISH assay has the potential to be a clinical assay for aGVHD prognosis, but significant refinements are necessary to achieve this milestone. The potential differences between the NanoString GeoMx and RNA-ISH include the overlapping signal of multiple USP17L isoforms that are not easily resolved by RNA-ISH, the higher sensitivity of the barcode sequencing-based RNA detection of GeoMx, and the ability to perform regional analysis of gene expression to account for tissue heterogeneity with GeoMx. Furthermore, constitutive staining in nonhematolymphoid stromal cells, the archival nature of tissue, and whole tissue ISH estimation compared with focal RNA sampling could contribute to the inability of RNA-ISH alone to predict aGVHD prognosis. Improvements in RNA-ISH probe design and refinements in regional analysis with digital imaging platforms are potential paths to overcome these limitations. In addition, combinational approaches using RNA-ISH with USP17L protein-dependent IHC staining, or immunofluorescence ISH, should be investigated for improved assay development.23,24
In conclusion, spatial transcriptomics on intestinal tissue obtained at diagnosis of lower intestinal aGVHD demonstrated distinct gene expression patterns that are associated with treatment response to corticosteroids. Future studies are needed to validate and expand on this differential expression, but the current study highlights how this platform can be used to identify potential tissue-based markers for the purpose of risk stratification. The prognostic value of UPS17L expression needs to be further evaluated in the context of established clinical risk factors and biomarkers.
Acknowledgments
The authors thank Danielle Bestoso for administrative support. B.K.P. acknowledges constructive input from Aliva Nayak, Peter Richieri, Katherine H. Xu, and Eunae You.
This study was supported by National Institutes of Health, National Cancer Institute grant U01CA228963 (D.T.T), the Rosalie H. Hornblower Endowed Research Fund in Bone Marrow Transplant (Z.D.), and the Wernick Family Fund (Z.D.).
Authorship
Contribution: B.K.P., Y.-B.C., D.T.T., and Z.D. conceptualized the study; B.K.P., M.J.R., D.T.T., and Z.D. performed formal analysis; B.K.P., Z.D., E.R.L., Y.S., C.L., and V.D. performed investigations; B.K.P., M.J.R., E.R.L., C.L., L.T.N., and M.J.A. performed methods; V.D., Y.-B.C., and Z.D. obtained resources; B.K.P., Y.-B.C., D.T.T., and Z.D. wrote the article; B.K.P., M.J.R., E.R.L., Y.S., and C.L. performed visualization; L.T.N., D.T.T., and Z.D. supervised the study; D.T.T. and Z.D. performed project administration; and D.T.T. and Y.-B.C. acquired funding.
Conflict-of-interest disclosure: D.T.T. has received in kind research support and a speaking honorarium from NanoString Technologies, whose technology was used in this article; receives research funding from ACD-Biotechne, whose technology was used in this work; has received consulting fees from Moderna, ROME Therapeutics, Tekla Capital Management, Ikena Oncology, Pfizer, Merrimack Pharmaceuticals, Ventana Roche, Foundation Medicine, Inc, and EMD Millipore Sigma, which are not related to this work; is a founder and has equity in ROME Therapeutics, PanTher Therapeutics, and TellBio, Inc, which are not related to this work; and receives research funding from Incyte Pharmaceuticals and AVA LifeScience, which are unrelated to this work. Z.D. receives research support from Incyte Corp, Regimmune Corp, and Taiho Oncology, Inc, and has received consulting fees from Kadmon Corp, Omeros Corp, Incyte Corp, MorphoSys AG, Inhibrx, PharmaBiome AG, and Ono Pharmaceutical. Y.-B.C, has received consulting fees from Incyte, Takeda, Magenta, Moderna, Daiichi, Actinium, Equilium, and Celularity. The remaining authors declare no competing financial interests.
Correspondence: David T. Ting, Mass General Cancer Center, 149 13th St, Room 6003, Charlestown, MA 02129; e-mail: dting1@mgh.harvard.edu; and Zachariah DeFilipp, Mass General Cancer Center, 55 Fruit St, Boston, MA 02114; e-mail: zdefilipp@mgh.harvard.edu.
References
Author notes
∗Y.-B.C., D.T.T., and Z.D. jointly supervised this work.
For original data, please contact corresponding author Zachariah DeFilipp (zdefilipp@mgh.harvard.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.