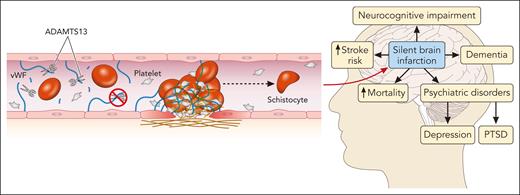
In this issue of Blood, Chaturvedi et al show that silent cerebral infarction (SCI) is a common finding in patients in clinical remission with immune-mediated thrombotic thrombocytopenic purpura (iTTP). Furthermore, SCIs are associated with both mild and major cognitive impairment.1
SCI is defined as abnormal finding in a magnetic resonance imaging (MRI) of the brain in the setting of a normal neurologic examination without a history or physical findings associated with an overt stroke. SCIs have been described in other diseases such as sickle cell disease and systemic lupus erythematous (SLE). In this study, the authors evaluated 42 patients with iTTP in clinical remission prospectively with MRI and the National Institutes of Health ToolBox Cognition Battery. SCI was present in 50% of patients, and those with SCI had higher rates of cognitive impairment. Increasing age and a history of stroke were associated with SCI in a logistic regression model, adjusted for the number of iTTP episodes and hypertension.
iTTP previously was considered an acute illness in which the only concern after diagnosis was the risk of future relapses. Studies have shown several complications beyond the risk of relapse. Patients with iTTP have a high risk of chronic medical problems that significantly impact their quality of life. These include hypertension, SLE, depression (19%),2 headaches,3 and neurocognitive impairment.4,5 These disorders appear to develop while patients are in clinical remission. Reeves et al evaluated 101 patients with iTTP during remission and found that their rates of fatigue and cognitive impairment were much worse than the rate in the US population but similar to the rates of chronic autoimmune disorders such as SLE. The severity of the symptoms was not associated with a history of relapses, and most of the patients (62%) with severe impairment by both fatigue and cognitive measures were >5 years from their most recent episode.6 The accumulation of these chronic medical problems leads to significantly increased mortality of patients with iTTP. iTTP survivors have a mortality rate nearly twofold higher than expected based on the US reference population, mainly due to cardiovascular disease and iTTP recurrence.7
The exact mechanism underlying these long-term complications is not fully understood. ADAMTS13 below the normal range that often occurs in patients with iTTP during remission may predispose patients to chronic microvascular injury and endothelial damage.8 The Rotterdam study found that even within the normal range of ADAMTS13 activity (≥60%), people aged ≥60 years with ADAMTS13 activity in the lowest quartile of normal (<80%) had a twofold higher risk of stroke than people in the highest quartile.9 Moreover, patients with acute ischemic stroke and chronic cerebrovascular disease had lower levels of ADAMTS13 than healthy individuals and a higher von Willebrand factor (VWF) level: ADAMTS13 ratio correlated with stroke severity.10 These studies and others suggest that ADAMTS13 may have physiologic antithrombotic properties through its cleavage of VWF. The study by Chaturvedi et al supports this hypothesis; ADAMTS13 data were available for the 12 months preceding research evaluation in 37 patients. The average remission ADAMTS13 activity was lower in patients with SCI when compared with those without SCI. As the authors appropriately point out, this does not represent the entire evaluation period. More data are needed to confirm the relationship between ADAMTS13 activity in remission and SCI in iTTP (see figure).
Thrombotic microangiopathy and the brain. In iTTP, autoantibodies against ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) prevent the cleavage of VWF multimers, leading to microthrombus formation and endothelial damage and resulting in ischemic end-organ damage, particularly in the central nervous system. SCIs are common in patients with iTTP while in remission and carry significant risks of associated morbidity and mortality. PTSD, posttraumatic stress disorder. Professional illustration by Patrick Lane, ScEYEnce Studios.
Thrombotic microangiopathy and the brain. In iTTP, autoantibodies against ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) prevent the cleavage of VWF multimers, leading to microthrombus formation and endothelial damage and resulting in ischemic end-organ damage, particularly in the central nervous system. SCIs are common in patients with iTTP while in remission and carry significant risks of associated morbidity and mortality. PTSD, posttraumatic stress disorder. Professional illustration by Patrick Lane, ScEYEnce Studios.
This study brings up several questions about the optimal management of patients with iTTP after their initial diagnosis: (1) What is the ideal range for ADAMTS13 activity in remission, both for relapse prevention and neurocognitive complications? (2) What other biomarkers can be used to better predict which patients will have SCI? (3) Will patients treated with caplacizumab have fewer and/or less severe long-term complications by stopping the thrombotic microangiopathy sooner? (4) Will other therapies targeting ultralarge VWFs, such as N-acetylcysteine or even recombinant ADAMTS13, prevent microthrombosis during remission? Future prospective studies are required to answer these questions. But certainly, the results of this study highlight the importance of monitoring patients with iTTP during remission to monitor for complications of the disease, screen for other cardiovascular risk factors, monitor the ADAMTS13 activity, and consider the need for preemptive therapy with rituximab or other immunosuppressant when ADAMTS13 activity is persistently low. Rituximab has been shown to prevent relapses; however, its impact on the long-term complications of the disease needs to be determined.
Conflict-of-interest disclosure: The author is on the advisory boards of and is a consultant for ArgenX, Sanofi, and Takeda.