The administration of Chimeric Antigen Receptor T-cell (CAR-T) therapy for relapsed refractory multiple myeloma (RRMM) predominantly takes place in the inpatient setting to monitor for adverse effects like cytokine release syndrome (CRS) and neurotoxicity leading to increased healthcare utilization, infection risk and patient discomfort. Shifting the administration model to the outpatient setting may improve patient experience, optimize hospital bed utilization and decrease costs, but requires an objective and reliable method for CRS detection to monitor patients remotely and safely. The feasibility of using a wearable device for detecting CRS following CAR-T therapy in RRMM was compared to standard of care (SoC) nursing-recorded vital signs in this investigator initiated clinical trial (IIT).
Patients wore a wearable device that collected continuous measures of temperature, pulse, respiratory rate, and O2 saturation in addition to SoC while receiving CAR-T therapy from the day infusion to discharge. Changes in vital signs captured by the device were analyzed based on 2 thresholds: (i) CRS timestamps were tagged in wearable data when pt's temperature breached a fixed threshold of 38 0C ( Tf) defined as fever by the American Society of Transplantation and Cellular Therapy (ASTCT), and (ii) when pt's temperature breached an individualized threshold of 2 standard deviations above their baseline temperature ( Ti) [see Figure 1]. Outcomes were time to first detection of CRS in wearable data vs. SoC. Wearable adherence was the duration pts wore the device over the total monitoring period.
To date, 34 pts were screened, 28 enrolled (82.3% uptake), 1 was excluded from the dataset because of concurrent COVID-19, and 2 pts with CRS were excluded from time to detection comparison because of wearable adherence <50% during high-risk windows (defined as the time period where CRS is expected per known data for the CAR-T product). Max CRS grades were 1 (17 pts), 2 (1 pt), 3 (2 pts), and 4 (1 pt); 5 pts did not have clinical CRS. Patients wore the device for a median of 13 (12-15) days out of a median of 15.5 (14-17) days of inpatient admission for CAR-T infusion. Median individualized temperature threshold was 37.4 (37-37.6) C. Wearable adherence was 64 (51 - 77) % and 72 (57 - 87) % for the overall monitoring and high-risk periods, respectively. The wearable detected the initial CRS events at a median of 22 (-43 - 113.5) min earlier than SoC with Tf and a median of 184 (53 - 312) mins earlier than SoC with Ti method. In the no CRS subgroup, false CRS detection occurred in 1 pt with the Tf and 2 pts with the Ti method.
Preliminary results suggest that time to CRS detection by the wearable device preceded SoC detection by a median of 103 minutes corroborated by both threshold methods. Temperature thresholds calibrated to the baseline temperature of individual patients facilitate earlier detection of CRS than fixed thresholds per clinical/ASTCT criteria. While the low false detection rate in is encouraging, these may also potentially be attributed to subclinical events. Both pts who had “false positive” triggers breached their individualized temperature thresholds via device readings while only recording low-grade temperatures that did not meet clinical CRS criteria per ASTCT, indicating possible capture of subclinical events. The study showcases the utility of continuous remote patient vital signs monitoring by wearable devices in efficiently capturing CRS, including subclinical events, earlier than SoC. Objective and reliable CRS monitoring may help safely transition workflows to outpatient CAR-T administration models. Analysis of trends in cytokine biomarkers for early CRS detection signal(s) is currently underway. Cytokine signals, alone and in combination with device data, are planned to be incorporated into machine learning models to build a CRS prediction index in future analyses.
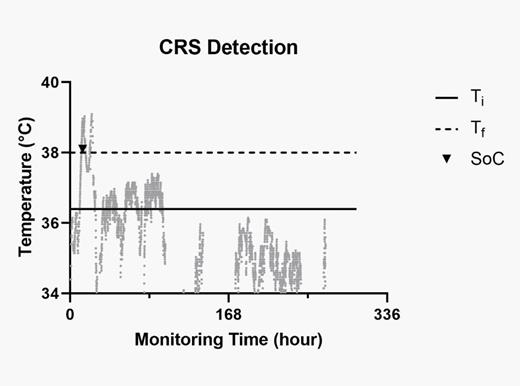
Figure 1: Snapshot in time of the continuous temperature data generated by the wearable device after CAR-T infusion showing capture of cytokine release syndrome.
– Ti (solid line) represents the median baseline temperature of the patient over the entire duration of the data capture by the wearable device
– Tf (dotted line) represents the temperature threshold of 38 0C, the clinical definition of fever as per ASTCT
– Black diamond: clinical CRS event, when temperature >38 0C as recorded by nursing
Disclosures
Zahradka:Current Health, A Best Buy Inc: Current Employment, Current holder of stock options in a privately-held company. Wilkes:Current Health, A Best Buy Company: Consultancy, Current Employment, Current holder of stock options in a privately-held company. Calafat:Current Health, a Best Buy Company: Current Employment, Current equity holder in publicly-traded company. Sanchez:Janssen Pharmaceuticals: Consultancy, Honoraria. Richard:Heidelberg Pharma: Research Funding; C4 Therapeutics: Research Funding; Bristol Myers Squibb: Honoraria; Janssen: Honoraria. Richter:Abbvie: Consultancy; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers-Squibb: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Rodriguez:Janssen, Takeda, Bristol Myers Squibb, Amgen, Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees. Cho:Bristol Myers-Squibb: Research Funding; Takeda, Inc.: Research Funding. Chari:Janssen: Consultancy, Other: Advisory Board, Research Funding; Celgene/BMS: Consultancy, Other: Advisory Board, Research Funding; Amgen: Consultancy, Other: Advisory Board, Research Funding; Karyopham: Other: Advisory Board; Sanofi: Other: Advisory Board; Seattle Genetics: Other: Advisory Board, Research Funding; Millenium/Takeda: Consultancy, Research Funding; Antengene: Consultancy; Glaxo Smith Kline: Other: Advisory Board; Secura Bio: Consultancy, Other: Advisory Board; Shattuck Labs: Other: Advisory Board; Genentech: Other: Advisory Board; AbbVie: Other: Advisory Board. Jagannath:Legend Biotech: Consultancy; Caribou: Consultancy; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy; Janssen: Consultancy; Bristol Myers Squibb: Consultancy; Karyopharm: Consultancy. Rossi:Sanofi: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees; JNJ: Membership on an entity's Board of Directors or advisory committees. Parekh:Celgene/BMS Corporation: Research Funding; Amgen: Research Funding; Karyopharm Therapeutics: Research Funding; Grail, LLC: Membership on an entity's Board of Directors or advisory committees; Caribou Biosciences: Research Funding.