Hemolytic anemias, such as sickle cell disease (SCD), are a group of acute and acquired conditions characterized by the accelerated destruction of red blood cells (RBC). Intravascular hemolysis (IVH), or RBC lysis within the bloodstream, incurs the release of damage-associated molecular patterns (DAMPs), trigging cell activation and immune responses. Caspase-1 (Casp-1), a protease enzyme, plays a critical role during inflammatory processes by initiating programed cell death and promoting the cleavage of pro-interleukin (IL)-1β into its active form, IL-1β, through inflammasome assembly.
Here, we aimed to elucidate the complex mechanisms involved in IVH-induced inflammation, leukocyte-endothelium interactions, and microvascular dynamics. Acute IVH was induced in C57BL/6 mice (HEM) by the injection of sterile water (i.v., 150 µl), and control mice received i.v. saline (CON) (n=3-8 per group). Intravital microscopy was performed at 15 min post IVH induction on the cremaster muscle of mice to quantify venular leukocyte (WBC) rolling and adhesion, and non-invasive laser Doppler flowmetry (LDF) measured blood perfusion in the murine pelvis microvasculature. Blood samples were collected at 1-hour post-IVH for ELISA and flow cytometry analyses. Immortalized human endothelial cells (HUVEC; n=7 per group) were stimulated with heme or S100A8 for 3 hours.
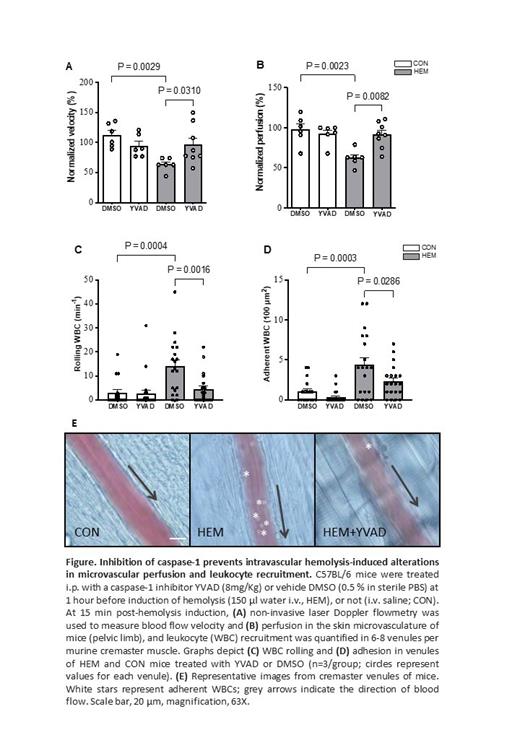
HEM mice demonstrated significantly increased plasma heme (P<0.0001) and cell-free hemoglobin (P<0.001), in association with significantly decreased plasma haptoglobin (P<0.05), compared to CON mice, thereby confirming the occurrence of IVH. Furthermore, HEM mice exhibited significantly increased plasma concentrations of the S100A8 alarmin (P<0.001) and IL1-β (P<0.001), as well as augmentations (P<0.001) in plasma TNF-ɑ and IL-6 levels. Flow cytometry was used to measure active Casp-1 in neutrophils and monocytes of mice using the FAM-FLICA probe. FLICA staining was significantly higher in the neutrophils (P<0.05) and monocytes (P<0.05) of HEM mice, compared to CON mice, implying Casp-1 activation in these cell subsets. WBC-endothelium interactions are a hallmark of vaso-occlusion in SCD. HEM mice exhibited elevated venular WBC rolling and adhesion to the endothelium, compared to CON mice (P<0.001), as previously shown (Almeida et al., 2015; PMID: 26019278). In addition, IVH significantly reduced the velocity of microvascular blood flow in mice (P<0.001), and impaired perfusion (P<0.001). To address the role of IVH-induced Casp-1 activation in microvascular dynamics, mice received i.p. administrations of the Casp-1 inhibitor, YVAD, at 1 hour prior to induction of IVH, or not, and were subjected to intravital microscopy or LDF. Interestingly, the inhibition of Casp-1 significantly abrogated IVH-induced WBC rolling (P<0.01) and adhesion to vessel walls (P<0.05) in HEM mice and significantly improved microvascular blood perfusion and flow velocity (P<0.05), suggesting a role for Casp-1 activation in the WBC-endothelium and occlusive interactions observed (See Figure).
We then evaluated how heme and S100A8 may induce endothelial cell activation, as these DAMPs were found elevated in the plasma of HEM mice. As expected, compared to unstimulated HUVEC, heme induced endothelial cell activation in vitro, as shown by higher expressions of ICAM-1 (P<0.05), VCAM-1 (P<0.01) and E-selectin (P<0.01), and increased Casp-1 activity (P<0.01). In turn, S100A8 alone significantly elevated ICAM-1 (P<0.05) and E-selectin (P<0.01) expressions on HUVEC, as well as IL-1β release (P<0.001), however no significant increase in Casp-1 activity was observed. HUVEC, when pre-treated with YVAD, displayed a significant reduction in S100A8-induced E-selectin expression (P<0.05), but neither ICAM-1 nor VCAM-1 expression were significantly decreased. In contrast, no changes in the expressions of these adhesion molecules were observed in heme-stimulated cells after YVAD treatment.
Taken together, these findings suggest that targeting caspase-1 activation and its associated pathways may represent a potential therapeutic intervention to mitigate the adverse inflammatory and microvascular effects of IVH, a disease mechanism that occurs in severe hemolytic anemias such as SCD.
Disclosures
Gotardo:Novartis Pharma AG: Other: Post Doctoral Fellowship Grant. Costa:Pfizer: Consultancy; Novartis Pharma AG: Honoraria. Conran:Novartis Pharma AG: Research Funding.