Introduction
The advent of venetoclax (VEN) based chemotherapy has revolutionized the treatment of elderly acute myeloid leukemia (AML), with improvement in remission rates from historical 10%-20% to 60%-70%. However, remission duration is short-lived in patients (pts) with high-risk AML with consequent dismal outcome. As our knowledge of genomic landscape of AML expands and more immunotherapies become available, exploration of the immune tumor microenvironment (TME) of AML may allow identification of subsets of pts that may benefit from immune based therapy. We conducted an investigator initiated prospective study to evaluate TME of pts receiving VEN plus hypomethylating agent (HMA; azacitidine or decitabine) combination. We hypothesize that these pts will cluster into immune-infiltrated vs. immune-depleted sub-types by high-definition immune cell profiling.
Methods
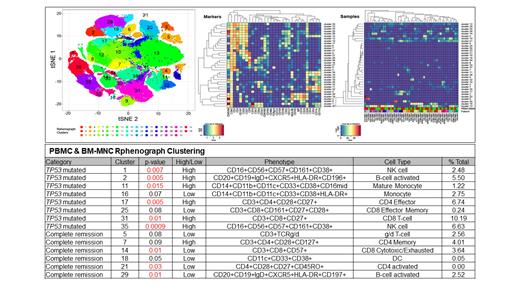
In 26 newly diagnosed AML pts, who were planned to receive VEN plus HMA therapy, we isolated bone marrow (BM) and peripheral blood (PB) derived mononuclear cells (MC) by density gradient centrifugation and cryopreserved. We performed CyTOF (cytometry time-of-flight) analysis to study their immune signatures. Thawed cells were labeled with metal-conjugated antibodies that bind CD33, CD196, CD19, CD127, CD11b, CD4, IgD, CD11c, CD14, CD56, CD123, TCRgd, CD185/CXCR5, CD45RA, CD27, CD28, CD66b, CD183 (CXCR3), CD161, CD45RO, CD197 (CCR7), CD8a, CD25, CD3, CD20, CD38, HLA-DR, CD194 (CCR4), CD57, CD16 and CD45. We performed a Rphenograph clustering on 25 paired BMMC and PBMC samples (one patient only had PBMC) to create 35 unique clusters for each ( Figure 1). Then we broke down the distribution of cluster by two different groupings: pts with TP53 mutation (m) and those who achieve complete remission. Statistical significance of clusters calculated using t-test. We performed Pearson correlation test on the paired BMMC and PBMC samples to assess correlation between BMMC and PBMC samples.
Results
Baseline characteristics
The median age of the pts was 74 years (range, 53-88), and 17 (65%) pts were male. Ten (37%) pts had secondary AML, and 12 (46%) pts had ELN adverse risk disease (n=6; TP53m). There was no significant difference in median age (p= >0.99), proportion of pts with secondary AML (p= 0.64), acute myelomonocytic leukemia (p= 0.14) white blood cell count (WBC) ≥ 100 (10 9/L) (p= >0.99), and frequency of commonly occurring co-mutations ( ASXL1 [p=0.35]), FLT3 ITD [p=0.54], splicing [p=0.29], IDH1/2 [p=0.28], DNMT3A [p=0.29], NPM1 [p=0.29], TET2 [p=0.35]) in TP53m and TP53 wild-type group.
Response
12/20 (60%) evaluable pts achieved complete remission with or without count recovery (CR/CRi). There was no significant difference in median age (p= 0.06), proportion of pts with secondary AML (p= 0.16), therapy related AML (p= 0.60) WBC ≥ 100 (10 9/L) (p= 0.40), and frequency of commonly occurring mutations ( TP53 [p=0.60], ASXL1 [p > 0.99]), FLT3 ITD [p=0.54], splicing [p > 0.99], IDH1/2 [p=0.60], DNMT3A [p=>0.99], NPM1 [p=0.61], TET2 [p >0.99]) in CR/CRi and no-CR/CRi group.
CyTOF analysis and Rphenograph clustering on 25 paired BMMC and PBMC samples
We found significant clustering with high expression of B-cell activated (cluster 2; p= 0.005), NK cell rich (cluster 1; p= 0.005, cluster 35; p= 0.0009), CD8 T-cells (cluster 31; p= 0.01) and CD4 effector cells (cluster 17; p= 0.005) phenotype among TP53m AML ( Table 1). Among pts who achieved CR/CRi, significant clustering with low expression of B-cell activated (cluster 29; p= 0.01), CD8 cytotoxic (cluster 14; p= 0.01) CD4 activated (cluster 21; p= 0.03) phenotypes observed.
Correlation between BMMC and PBMC clustering
Among clusters which showed significance for a particular phenotype, we performed Pearson correlation (R 2) and observed strong correlation between BMMC and PBMC samples clustering in cluster 1 (R 2 = 0.99), cluster 2 (R 2 = 0.95), cluster 17 (R 2 = 0.96), cluster 21 (R 2 = 0.99) and cluster 29 (R 2 = 0.93).
Conclusion
In this single institutional study using CyTOF analysis we have identified novel clustering with immune- infiltrated phenotype among pts with TP53m AML and conversely immune-depleted phenotype among AML pts who responded to VEN plus HMA. Moreover, we found strong correlation in CyTOF analysis conducted from BMMC and PBMC samples. Future studies with larger sample size needed to validate our findings.
Disclosures
Foran:BeiGene: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Actinium: Research Funding; Kura: Research Funding; Sellas: Research Funding; Roivant: Research Funding; Novartis: Research Funding; Celgene: Research Funding; Astellas: Research Funding; NCI: Membership on an entity's Board of Directors or advisory committees; CTI: Membership on an entity's Board of Directors or advisory committees. Murthy:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Senti Biosciences: Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Bavarian Nordic: Membership on an entity's Board of Directors or advisory committees. Alkhateeb:Mayo Clinic: Current Employment. Patnaik:Epigenetix: Research Funding; Kura: Research Funding; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; StemLine: Research Funding. Shah:Astellas: Research Funding; MRKR Therapeutics: Research Funding; Celgene: Research Funding; AbbVie: Research Funding.