Background: A high incidence of severe toxicities especially grade 3/4 immune effector cell-associated neurotoxicity syndrome (ICANS) and cytokine release syndrome (CRS), need for hospitalization, and the cost of supportive care is an hindrance to the implementation of CAR-T cell therapy for low and middle income countries (LMICs). To address these limitations, we designed a novel humanized CD19 CAR and demonstrated a favorable balance of efficacy to toxicity in the preclinical model and in the first-in-human Phase I study (Dwivedi et al. Mol Cancer Ther. 2021, Karulkar et al. ASH 2022). Here, we quantified the healthcare resource utilization (HCRU) and associated cost of clinical management of Actalycabtagene autoleucel (Actaly-cel) in India from an ongoing Phase I/II study in B-cell malignancies.
Methods: This is a retrospective analysis of the HCRU costs of patients with r/r B-cell malignancies treated on Phase I/II study with Actaly-cel at 3 centers in India between June 2021 through July 2023. The key components of HCRUs are defined in Table 1 and were quantified from the electronic records from the time of enrolment till their last follow-up. Once enrolled, bridging therapy was allowed at investigator's discretion. After lymphodepletion (LD) with a fludarabine plus cyclophosphamide (FC) regimen for 3 days, patients received Actaly-cel at a target dose ≥ 5 million CAR-T cells/kg (upper limit; total 2 x10 9). The patients were monitored for toxicities particularly CRS, ICANS and cytopenias, as inpatient (non-HEPA filtered rooms) for at least a week. CRS and ICANS were graded as per ASTCT grading system and cytopenias as per CTCAE v5.
Results: Total 59 patients were enrolled in this study and 47 patients received the infusion. Thirty seven patients (r/r lymphoma; n=28, r/r B-ALL; n=9) who completed 28 days post-infusion were evaluable for HCRU analysis. The median age was 37 years (16-71). The median follow-up of the cohort was 60 days (21-644); 19% (7/36) and 11% (4/36) patients completed 6 months and 12 months follow-up respectively.
Among all patients (n=37) who received LD therapy, 46% of lymphoma patients received LD in the outpatient setting while 78% of B-ALL patients required hospitalization for LD. The median hospitalization period post Actaly-cel infusion was only 8 days (range: 7-17 days) in the lymphoma cohort, and 13 days (range: 7-19 days) for B-ALL patients. Importantly, none of the lymphoma patients required ICU admission, and only one B-ALL patient required ICU admission for management of adverse events.
In the lymphoma cohort, 54% (15/28) of patients developed Grade 1/2 CRS and none had grade >/=3. A single dose of tocilizumab was given to 36% (10/28) of patients and only 4% (1/28) received tocilizumab plus corticosteroids. None of the patients required vasopressor support.
Majority of B-ALL patients developed CRS. The incidence and severity of CRS was as follows: G1; 44% , G2; 11% and G3; 22%. There were no grade 4/5 CRS events. While 11% (1/9) of patients received a single dose of tocilizumab, 44% (4/9) received tocilizumab in combination with corticosteroids. Vasopressors were required in 22% of patients.
None of the patients developed ICANS of any grade .
Most patients (lymphoma and B-ALL) developed hematological toxicities(Table 1). The severity of Grade 3-4 was more prevalent in the B-ALL cohort. IVIG was administered to 25% of lymphoma and 22% of B-ALL patients. The induction and severity of the above toxicities were not dependent on the Actaly-cel dosage (median: 9 x 10 6 ;range: 2 x 10 6-21 x 10 6 CAR-T cells/kg).
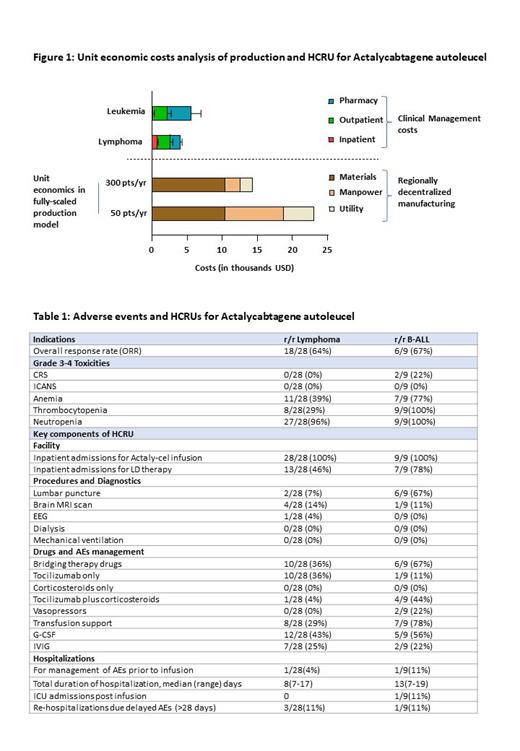
The projected production cost of Actaly-cel in the regional decentralized manufacturing model at the scale of 300 patients/year is ~15,000 USD per patient. The mean cost of HCRU for clinical management (until last follow-up) per patient was ~ 4,400 USD in an academic centre (lymphoma; ~4,000 USD, B-ALL; ~5,565 USD) and interestingly, only about 14% of these costs were incurred due to inpatient management (Figure 1).
Conclusions: Actaly-cel was efficacious, with no incidence of ICANS and manageable CRS across a wide dose range. More importantly, this study reported low HCRUs due to negligible ICU admission and shorter duration of hospitalization, ensuring potential usage of Actaly-cel in outpatient settings, and ultimately improving the access and feasibility of CD19 CAR-T cell therapy in resource constrained settings such as LMICs.
Disclosures
Karulkar:Immunoadoptive Cell Therapy Private Limited: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Kalra:Immunoadoptive Cell Therapy Private Limited: Current Employment. Ravikumar:Immunoadoptive Cell Therapy Private Limited: Current Employment. Ghandade:Immunoadoptive Cell Therapy Private Limited: Current Employment. Patil:Immunoadoptive Cell Therapy Private Limited: Current Employment. Shah:Immunoadoptive Cell Therapy Private Limited: Current Employment. Firfiray:Immunoadoptive Cell Therapy Private Limited: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Patil:Immunoadoptive Cell Therapy Private Limited: Current Employment. S:Immunoadoptive Cell Therapy Private Limited: Current Employment. Thorat:Immunoadoptive Cell Therapy Private Limited: Consultancy. Shah:Immunoadoptive Cell Therapy Private Limited: Consultancy, Other: Scientific Advisory Board; CARGO: Consultancy; VOR: Consultancy, Research Funding; Lentigen: Research Funding. Neelapu:Fosun Kite: Consultancy, Other: Advisory board member; Sana Biotechnology: Consultancy, Other: Advisory board member, Research Funding; Precision Biosciences: Research Funding; Synthekine: Consultancy, Other: Advisory board member; Orna Therapeutics: Consultancy, Other: Advisory board member; Caribou: Consultancy, Other: Advisory board member; Astellas Pharma: Consultancy, Other: Advisory board member; Morphosys: Consultancy, Other: Advisory board member; Janssen: Consultancy, Other: Advisory board member; Chimagen: Consultancy, Other: Advisory board member; Immunoadoptive Cell Therapy Private Limited: Consultancy, Other: Scientific Advisory Board; Takeda: Consultancy, Other: Advisory board member; Carsgen: Consultancy; N/A: Patents & Royalties: Related to cell therapy and the safety switch described (intellectual property); Longbow Immunotherapy: Current holder of stock options in a privately-held company; Merck: Consultancy, Other: Advisory Board Member; Sellas Life Sciences: Consultancy, Other: Advisory board member; Athenex: Consultancy, Other: Advisory board member; Allogene: Consultancy, Other: Advisory board member, Research Funding; Incyte: Consultancy, Other: Advisory board member; Adicet Bio: Consultancy, Other: Advisory board member, Research Funding; Kite, A Gilead Company: Consultancy, Other: Advisory Board Member, Research Funding; Bristol Myers Squibb: Consultancy, Other: Advisory Board Member, Research Funding; Bluebird Bio: Consultancy, Other: Advisory board member. Jain:Intas Pharmaceuticals: Research Funding; Zydus Pharmaceuticals: Research Funding; ImmunoACT: Research Funding. Purwar:Immunoadoptive Cell Therapy Private Limited: Current Employment, Current equity holder in private company, Patents & Royalties.