Introduction: Patients with SARS-CoV-2 show significantly elevated plasma levels of von Willebrand factor (VWF) and increased thrombosis. However, the molecular mechanism underlying increased plasma levels of VWF in COVID-19 patients is not fully understood.
Methods. Drosophila genetic screen, recombinant expression, endothelial culture, confocal microscopy, flow cytometry, and a microfluidic assay were employed for the study.
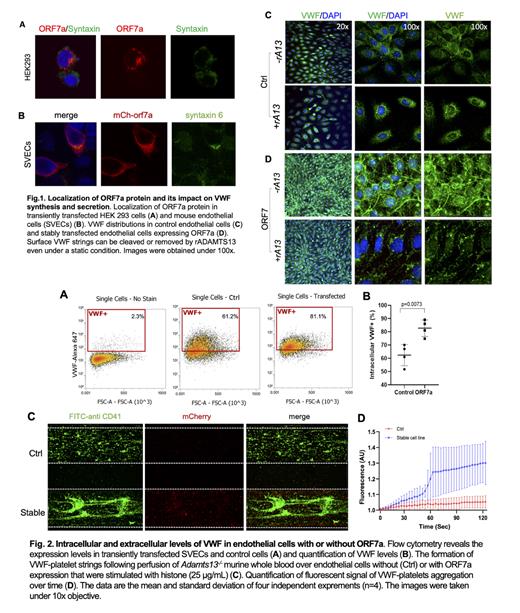
Results. Drosophila genetic screen for SARS-CoV-2 proteins allowed us to identify Orf7a to be the key factor that induces Hml (drosophila homolog of human VWF)-dependent hypercoagulability. Recombinant Orf7a tagged with mCherry (mCh) and FLAG was expressed transiently and stably in a murine endothelial cell line SVEC4-10 and a HEK293 cell line. The ORF7a protein is localized predominantly to the Golgi and trans-Golgi network (TGN) in HEK293 cells ( Fig. 1A) but shows its expression in perinuclear area and cell surface in SVEC4-10 cells ( Fig. 1B). Significantly higher numbers of VWF-strings were formed in the ORF7a transfected SVEC4-10 cell line under static state than in the non-transfected control cells. These cell surface or extracellular VWF strings were readily removed by a human recombinant ADAMTS13 ( Fig. 1C & 1D). Flow cytometry analysis revealed that significant higher levels of intracellular VWF signal in the cells following transient expression of Orf7a than in those non-expressed controls ( Fig. 2A & 2B). Microfluidic shear-based assay and confocal imaging analysis further demonstrated that dramatically increased rate and surface coverage of fluorescent labeled murine platelets over a histone-stimulated SVEC surface expressing Orf7 comparing with over the same cells without expression of Orf7, following perfusion of Adamts13 -/- murine whole blood (anticoagulated with PPACK) under arterial shear ( Fig. 2C & 2D). Using proteomics, we identified conserved host proteins ARF4, GBF1 and ERGIC-53 (a COPII pathway component) as direct interacting factors for Orf7a and demonstrated that these proteins have conserved roles in VWF secretion.
Conclusions:Our results demonstrate that ORF7a may upregulate VWF synthesis and secretion in endothelial cells, which enhances thrombus formation. Recombinant ADAMTS13 may rapidly remove endothelial surface ultra large VWF strings and exhibit therapeutic potential for COVID-19 associated thrombosis.
Disclosures
Zheng:GC Biopharma: Consultancy, Honoraria; TAKEDA: Consultancy, Honoraria; Stago: Consultancy, Honoraria; Apollo: Consultancy, Honoraria; Clotsolution: Other: Co-founder; Sanofi: Consultancy, Honoraria.