Introduction. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for coronavirus disease 19 (COVID-19), has led to a significant increase of morbidity worldwide. A recent meta-analysis showed a high case fatality rate in hospitalized patients with hematological malignancies and published reports indicated high rates also in chronic lymphocytic leukemia patients (CLL) patients.
The aim of our study was to evaluate the impact of COVID-19 in CLL patients treated with venetoclax.
Methods. The retrospective multicenter study included CLL patients treated since 2017 with venetoclax single agent until progression or toxicity or venetoclax plus anti-CD20 antibody (mainly rituximab as part of VR protocol for 24 months in relapsed/refractory patients or obinutuzumab as part of VO protocol for 12 months in untreated patients).
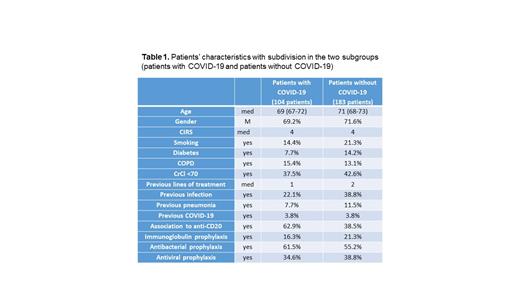
Results. We found 128 infections from SARS-CoV-2 in 104 patients out of 287 patients with CLL who received venetoclax. Basal characteristics of the 104 patients who experienced COVID-19 were compared to those of the 183 patients who did not experience the infection (Table 1). Patients of the first group did not show more comorbidities in terms of CIRS, nor renal and pulmonary impairment. Unexpectedly patients without COVID-19 experienced more previous infections in the 12 months before the beginning of venetoclax but a similar low rate of previous SARS-CoV-2 infection. They received a median of 2 different previous lines of treatment whereas patients with COVID-19 were exposed to a median of a single line of therapy. No significant differences were noted for prophylaxis with immunoglobulin, antiviral and antibacterial drugs.
In the group of COVID-19 a higher rate of patients received venetoclax plus anti-CD20 antibody (62.9% vs 38.5% of the group without COVID-19, p<0.001). The rate of vaccination was higher than 66% in both the groups with a median of 3 doses each. Prophylaxis with tixagevimab/cilgavimab was administered in less than 20% of the patients.
Analyzing the characteristics of the 128 infections we distinguished 73 grade 1-2 COVID-19 and 55 grade 3-4 COVID-19. Patients with grade 1-2 COVID-19 were positive for a median time of 10 days (range 5-97). No treatment was administered in 29.1% of the cases, nirmatrelvir/ritonavir was used in 23.6%, remdesivir in 18.2%. Regarding patients with grade 3-4 COVID-19 resulted positive for a median time of 21 days (range 5-120). Remdesivir was chosen as treatment in 48.9%, nirmatrelvir/ritonavir in 21.3%.
Univariate and multivariate analysis found that association to anti-CD20 was a risk factor for COVID-19 of any grade (OR 1.93) and of grade 3-4 (OR 2.96), conversely previous infection in the 12 months before the beginning of venetoclax was a protective factor for COVID-19 any grade (OR 0.42) and grade 1-2 (OR 0.32).
During COVID-19 Venetoclax was withdrawn in 38 (36.5%) patients, in 32 of whom venetoclax was administered together to anti-CD20 antibody; in 25/38 the discontinuation was only temporary. Thirty-five (33.6%) patients required hospitalization due to COVID-19, the median time of recovery was 15 days, and 8 (7.7%) patients needed intensive care unit admission.
Among the 104 patients with COVID-19, 18 patients died, 10 deaths were due to COVID-19: 7 were exposed to anti-CD20 antibody, 3 had a previous grade 1-2 COVID-19, but none experienced a SARS-CoV-2 infection before treatment with venetoclax. All were vaccinated with at least 3 doses except one patient who was not vaccinated but was infected and died in 2020. The rate of mortality due to COVID-19 was 9.6% considering patients who were infected by SARS-CoV-2, but 18.2% among patients with severe grade 3-4 COVID-19.
Conclusions. This is a real-life study on 287 patients affected by CLL treated with venetoclax with the aim to describe COVID-19 in this cohort. The analysis found over a third of patients infected by SARS-CoV-2, in 57% of the cases the infection was grade 1-2 with a mortality rate of almost 10%, but higher if COVID-19 was of grade 3-4 (18.2%). We confirmed the association of anti-CD20 antibody to venetoclax as a risk factor for SARS-CoV-2 infection and mortality rate in patients with CLL and severe COVID-19.
Disclosures
Visentin:CSL behring: Membership on an entity's Board of Directors or advisory committees; Takeda: Speakers Bureau; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Vitale:Janssen, Abbvie, Astra-Zeneca, Beigene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Sanna:Abbvie: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Astrazeneca: Consultancy, Speakers Bureau. Sportoletti:Abbvie, Janssen, Beigene, Astra Zeneca, Takeda, Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Tedeschi:Janssen: Speakers Bureau; Beigene: Speakers Bureau; Abbvie: Speakers Bureau; Astrazeneca: Speakers Bureau. Candoni:Incyte: Consultancy, Honoraria; Janssen: Honoraria; Astellas: Honoraria; Pfizer: Consultancy. Pagano:Janseen: Honoraria; Jazz: Honoraria; Gilead: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Menarini: Honoraria; Moderna: Honoraria; AstraZeneca: Honoraria.