Background
In randomized clinical trials (RCTs), patients (pts) with comorbidities or a very aggressive presentation of myeloma may be excluded. The results of RCTs may not be generalizable to pts in the real-world setting. Therefore, RCT data need to be backed up by results from real-world studies.
Methods
EMMY is a non-interventional, prospective study conducted in 73 IFM (Intergroupe Francophone du Myélome) sponsor sites in France. Any patient initiating treatment for multiple myeloma (MM) over a 3-month (mo) period, from October to December, is included, since 2017. It is a dynamic cohort with 900 pts on average, enrolled each year. Here, we present the results of NDMM pts for the first 4 years of inclusion (2017 to 2020). Pts with NDMM who receive front-line therapy with an autologous stem cell transplant (ASCT group) are described separately from non-transplant eligible pts (NTE group).
Results
1597 pts with NDMM were included in EMMY over the 2017-2020 period. Of these, 1036 were in the NTE group (64.9%) and 561 in the ASCT group (35.1%). Data are presented with a median follow-up of 23.7 mo (IQR 12.3 - 39.0).
ASCT group
These pts had a median age of 60.6 years (range 23.4 - 78.5). 5.2 % were older than 70 years at ASCT.
In routine practice, cytogenetic tests were carried out for 75.7% of pts, 19.6% of them harboured high-risk (HR) anomalies of t(4;14) or del(17p).
The period 2017-2020 was marked by a shift in induction treatment from Velcade Thalidomide dexamethasone (VTd) (n = 163, 29.1% of pts who received ASCT) to Velcade Revlimid dexamethasone (VRd) (n = 309, 55.1%).
Fifty-three (9.5%) pts received a double transplantation which was stable over time. 22.8 % of pts with HR anomalies received a double transplant.
NTE group
The 1036 NTE pts had a median age of 74.9 years (range 33.8 - 97.8), and more than 30.4% (n = 315) were aged ≥ 80 years. Among these pts, 65.9% were considered frail, with 33.6% having an Eastern Cooperative Oncology Group-Performance Status (ECOG-PS) ≥ 2 and almost half (47.1%) had comorbidities. Significant renal impairment (glomerular filtration rate [GFR] < 30 mL/min) was observed in 118 (13%) pts at treatment initiation.
A subgroup of 126 younger pts (age < 65 years) was considered ineligible for transplantation.
Regarding cytogenetics 56.4% of the pts were tested in regular practice (mean age 73.3 years).
NTE pts mostly received three main combinations: VRd (28.4% of pts, n = 294), Velcade Melphalan prednisone (VMp) (21.9% of pts, n = 21.9%) and Revlimid dexamethansone (Rd) (21.1%, n = 219). During the study period, VMp use decreased from 36.9% to 6.5% of pts, VRd use increased from 10.6% to 48.5% and Rd use remained constant, at around 20%. Other bortezomib-based regimens, including Velcade dexamethasone (Vd) and Velcade Cyclophosphamide dexamethasone (VCd), were used steadily, in approximately 12% and 7% of pts, respectively, over the period.
Survival data
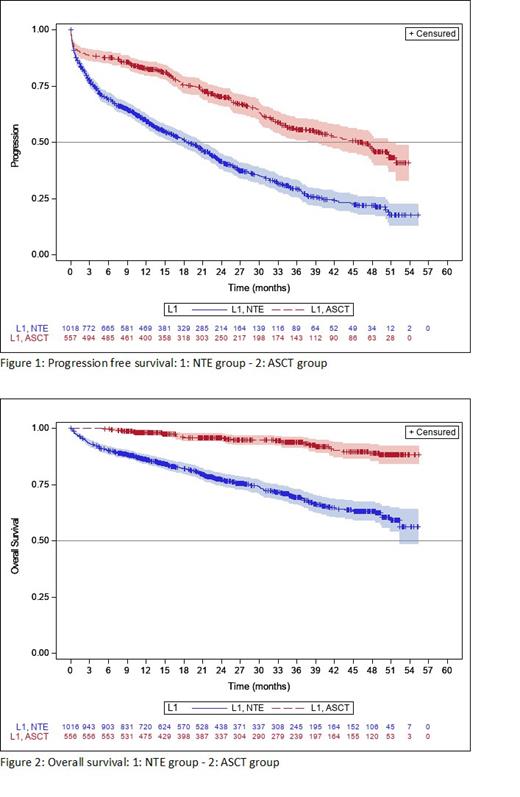
Median progression-free survival (mPFS) was 25.8 mo (95% CI: 23.5-29.1) in the overall NDMM population, 46.5 mo (95% CI: 37.8-50.6) in pts who received ASCT and 18.7 mo (95% CI: 16.3-20.8) in NTE pts ( P < 0.0001). (Figure 1).
The median PFS2 (mPFS2) was estimated at 50.6 mo (95% CI: 47.6-NA) in all pts with NDMM. mPFS2 was 36.4 mo (95% CI: 34.7-39.9) in NTE pts; it was not reached in the ASCT group.
The median overall survival (OS) had not yet been reached. The estimated OS rate was 72.9% (95% CI: 69.8-75.9) at 48 mo for the total NDMM population. (Figure 2).
AE leading to discontinuation.
139 pts experienced an adverse event (AE) leading to treatment discontinuation (6.8 % of ASCT pts and 14.5% in the NTE group). This occurred at a median of 4.3 mo after treatment initiation and was mainly reported with the most used agents (lenalidomide, bortezomib or melphalan). The most frequently reported AEs were neuropathy (n = 14; 10.1%), digestive disorders (n = 13; 9.4%), rash (n = 12; 8.6%), deterioration of general condition (n = 12; 8.6%), cardiovascular AEs (n = 10; 7.2%) and acute renal failure (n = 10; 7.2%).
Conclusion
The 2017-2020 period for pts with NDMM in France was marked by the expansion of the use of lenalidomide in first line for the majority of pts. A major difference in PFS and OS is still observed between ASCT and NTE pts. A shorter PFS than in IFM2009 trial (PFS 50 mo) was observed in EMMY ASCT pts.
Disclosures
Vincent:BMS, Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Financing meeting participation; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financing meeting participation; Pfizer: Other: Financing meeting participation. Decaux:Janssen, BMS, GSK, Sanofi, Takeda, Roche, Gilead: Honoraria. Perrot:Abbvie, Adaptive, Amgen, BMS, Janssen, Pfizer, Sanofi, Takeda: Honoraria. Macro:Janssen, Takeda: Honoraria, Other: Travel/accommodation, Research Funding; GSK, Sanofi: Honoraria. Karlin:AbbVie, Amgen, Celgene, Janssen, Sanofi, Takeda: Honoraria; Amgen, Celgene, GSK, Janssen, Takeda: Consultancy. Sonntag:Janssen, Takeda, BMS and Sanofi: Membership on an entity's Board of Directors or advisory committees. Mohty:JAZZ PHARMACEUTICALS: Honoraria, Research Funding. Frenzel:Pfizer: Consultancy, Other: Grant, Research Funding; CSL Berhing: Consultancy, Research Funding; Biomarin: Consultancy; Roche: Consultancy. Hulin:AbbVie: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Bristol Myers Squibb: Honoraria; Pfizer: Honoraria. Belhadj Merzoug:BMS: Research Funding; Amgen, BMS, Janssen, Sanofi: Honoraria; Amgen, Janssen, Pfizer, Sanofi, Takeda: Other: Travel Support.