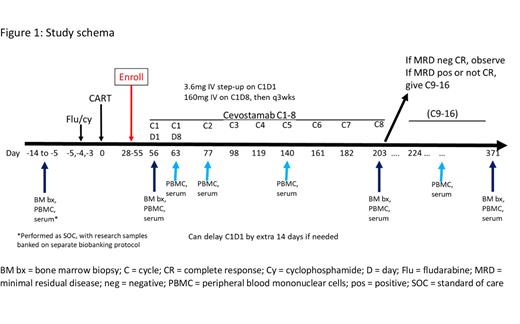
Background and significance: The BCMA-targeted CAR T products idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) are currently approved for relapsed/refractory multiple myeloma (RRMM) patients with ≥4 prior lines of therapy, including an IMID, proteasome inhibitor, and CD38 antibody. However, despite unprecedented response rates, CAR T cells are not curative in these late-line patients, even for those in complete remission. Mechanisms of resistance may include lack of persistence or poor function of persisting CAR T cells, as well as BCMA-low or -negative residual tumor cells that serve as a reservoir for relapse. FcRH5 is another MM cell surface antigen, with expression independent of BCMA. Cevostamab is an FcRH5-targeted, T cell-engaging bispecific antibody (bsAb) with demonstrated activity in RRMM, including in patients with prior BCMA-directed therapies (Trudel et al, ASH 2021, #157). We hypothesize that consolidating BCMA CAR T cell therapy with a bsAb targeting a different antigen may re-invigorate persisting CAR+ T cells against residual BCMA+ tumor cells, while also activating endogenous T cells against FcRH5+, BCMA-low/negative tumor cells, ultimately improving rates of sustained minimal residual disease (MRD)-negativity and durability of response. Study design and methods: This is a single-institution, investigator-initiated study (NCT05801939) sponsored by the University of Pennsylvania, with funding support from Genentech. Targeted population are patients with RRMM who have received a commercially available CAR T cell product (ide-cel or cilta-cel) according to the FDA label, within the past 8 weeks, with stable disease or better. Major inclusion criteria include absolute neutrophil count ≥ 1, hemoglobin ≥ 7, platelets ≥ 50, and creatinine clearance ≥ 30 ml/min. Major exclusion criteria include prior cytokine release syndrome or ICANS ≥ grade 3, or any grade hemophagocytic lymphohistiocytosis (HLH) or Parkinsonism, or any active infection. The study schema is shown in Figure 1. Cevostamab is initiated 8-10 weeks after CAR T cell infusion. This timepoint was chosen to allow recovery from acute CAR T cell-related toxicities, but while CAR T cells may still be detectable in circulation. Cevostamab is given at a step-up dose of 3.6mg intravenously (IV) on Cycle 1, Day 1 (C1D1), followed by full dose of 160mg IV on C1D8. Subjects are hospitalized for 48 hours after each C1 dose to monitor for CRS and ICANS. They then continue cevostamab every 3 weeks for total of 8 cycles. If they are in an MRD-negative complete response (CR) after 8 cycles (Adaptive Clonoseq assay, at 10e-5 sensitivity), they stop therapy and are observed. If not, or if bone marrow results are indeterminate, they get another 8 cycles of cevostamab, then stop and are observed. The primary endpoint is frequency of MRD-negative CR at 12 months post-CAR T cell therapy. Assuming a roughly equal proportion of patients enrolling after ide-cel and cilta-cel, the null hypothesis is that the true MRD-negative CR rate at 12 months is 35%. Twenty-six evaluable subjects will be accrued in a single-stage design. The null hypothesis will be rejected if 14 or more subjects meet the primary endpoint. This design yields a one-sided type I error rate of 0.05 and power of 0.84 for an exact test when the true 12-month MRD-negative CR rate is 60%. Secondary endpoints include feasibility, safety/tolerability, and other efficacy measures (overall and CR rates, PFS, OS). Exploratory endpoints include 1) impact of cevostamab on pre- and post-therapy frequency and phenotype of both CAR+ and CAR-negative T cells in blood and marrow, assessed by multiparameter flow cytometry; 2) pre- and post-therapy expression of BCMA and FcRH5 on myeloma cells (when available), serum concentrations of soluble BCMA and FcRH5, and relationship of these factors to clinical outcome measures, and 3) pre- and post-therapy genotypic and phenotypic make-up of bone marrow microenvironment, assessed by single cell RNA sequencing (scRNAseq) and multiparameter flow cytometry, and relationship of these factors to clinical outcome measures. Conclusions: This phase 2 study is exploring the efficacy, safety, and feasibility of cevostamab consolidation following BCMA-directed CAR T cell therapy for RRMM, with the goal of sequential T cell engagement against 2 different antigens to eliminate residual disease. Accrual started in July 2023.
Disclosures
Cohen:Ichnos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Abbvie: Consultancy; Arcellx: Consultancy; BMS/Celgene: Consultancy; Janssen: Consultancy, Research Funding; GSK: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Novartis: Patents & Royalties, Research Funding. Vogl:Genentech: Consultancy; Karyopharm: Consultancy; Active Biotech: Research Funding; Takeda: Consultancy, Research Funding; GSK: Consultancy; Sanofi: Consultancy. Garfall:BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety and Monitoring Board; GSK: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Legend: Consultancy, Honoraria. Ruella:NanoString: Consultancy, Research Funding; Beckman Coulter: Research Funding; GlaxoSmithKline: Consultancy; Bristol Myers Squibb: Consultancy; viTToria biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Scientific Founder, Research Funding; Bayer: Consultancy; AbClon: Consultancy, Research Funding. Stadtmauer:Amgen: Consultancy; Janssen: Consultancy; genmab: Consultancy; Abbvie: Consultancy, Research Funding; BMS: Consultancy.