Background Sickle cell disease (SCD) is an inherited disorder of red blood cells affecting millions of people worldwide. SCD is characterized by recurrent episodes of severe pain attacks, also called vaso-occlusive crisis (VOC), and are the most common reason for hospitalization. Approximately 90% of the hospital admissions are for pain treatment and the hospital readmission rate is alarmingly high, as 30-50% are re-admitted within 30 days. We previously were successful in developing machine learning models to predict pain using inpatient vital signs data as well as Apple Watch data in patients with SCD hospitalized for VOC. We now aimed to predict reutilization of care within 30 days in patients with SCD treated for a VOC.
Methods Patients with SCD aged 18 years and above, who were admitted for a VOC to the day hospital or Duke University Hospital between April and June 2022, were eligible for this study. Following informed consent, demographics, SCD genotype, details from the hospitalization such as length of stay, pain scores, as well as vital signs measured per standard of care were collected from the electronic medical records including blood pressure, pulse rate, respiratory rate, oxygen saturation and temperature. Baseline vital signs during regular visits were collected 6 months before and after the hospitalization. We used the values of nearest neighbors to fill in the empty entries. The vital signs over the whole hospital stay were averaged and those numbers were used as the predictor values for the model. The primary outcome was reutilization of care defined as readmission within 30 days to the day hospital and/or hospital. The predictors were used to fit 3 different machine learning classification models for the prediction of reutilization of care: random forest, logistic regression, gradient boosting. By fitting random forest model on the whole dataset, we were able to rank all the features using mean decrease in impurities. To avoid overfitting, we only used the best four predictors which were diastolic blood pressure, pulse rate, respiratory rate and pain score.The performance of the machine learning models was evaluated using the following metrics: accuracy, precision, recall, F1 score and area under the receiver-operating-curve (AUC).
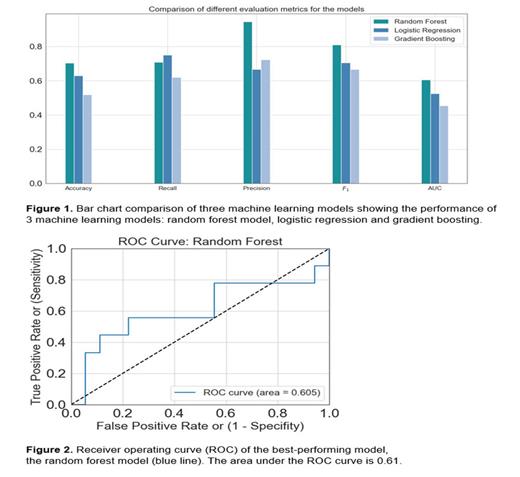
Results Eighteen participants with SCD were included in this study. The median age at inclusion was 30 years (IQR 22-34). The majority of the participants had SCD genotype HbSS (68%), and all were Black of African-American. There were 10 participants treated at day hospital (56%), while the other 8 participants were admitted to the hospital with a median length of stay of 7.5 days (IQR 2.5-10). After discharge, 15 participants sought medical care at least once within 30 days (83%); 8 were hospitalized (44%), and 13 were readmitted to the day hospital (72%). This pilot study consisted of 88 vital sign data points across the 18 patients. The metrics of our best-performing machine learning model, the random forest model, were: accuracy 70%, precision 0.94, recall 0.71, F1 score 0.81 and AUC 0.61 (Figure 1 and 2). The difference in precisions and recalls for all the models reflects the class imbalance in the dataset. To test how the model will perform for independent data sets, we used 5-fold cross-validation, and the cross-validation accuracy was 66% with a standard deviation of 7.7%.
Conclusion In this pilot study, our machine learning model was able to accurately predict health care reutilization within 30 days following discharge, with real-time vital signs data collected during clinic visits and hospital admissions in participants with SCD. Prediction of reutilization may help healthcare providers identify those at high risk and allow considerations for inpatient and outpatient strategies for patient management. Our next efforts include prediction of re-utilization from data collected from consumer wearables and in a larger number of participants.
Disclosures
Fijnvandraat:Sanofi: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy; Novo Nordisk: Consultancy, Research Funding; Sobi: Consultancy, Research Funding; CSL Behring: Research Funding. Shah:Agios Pharmaceuticals: Consultancy; Vertex: Consultancy; Bluebird bio: Consultancy; Alexion Pharmaceuticals: Speakers Bureau; Global Blood Therapeutics/Pfizer: Consultancy, Research Funding, Speakers Bureau; Forma: Consultancy.