Background: Venetoclax (VEN) in combination with hypomethylating agents (HMAs) is the standard of care for newly diagnosed AML patients who are older and determined to be unfit for intensive induction chemotherapy. The decreased intensity of HMA/VEN affords the possibility of outpatient management provided appropriate monitoring can be done for tumor lysis syndrome, persistent cytopenias, and infection risk inherent to initial AML directed therapy.
We evaluated the incidence of TLS, infection risk, and transfusion needs in patients with AML treated with HMA/VEN at our institution who initiated therapy as an inpatient versus outpatient to determine the safety and feasibility of initial outpatient HMA/VEN therapy.
Methods: This was a retrospective Lifespan IRB approved review of patients diagnosed with AML from 12/1/2016 to 12/1/2021. Patients who received HMA/VEN for AML, either as frontline or as relapsed/refractory therapy, were included. Patients' records were reviewed for demographic, leukemia specific, and patient specific outcomes. TLS was based on Cairo Bishop classification. Fisher exact/Pearson chi-squared and Mann Whitney U tests were used to determine statistical differences for categorical and continuous variables, respectively, while the log rank test was used for overall survival (OS).
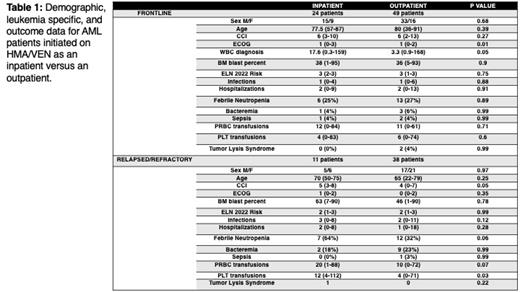
Results: Between 12/1/2016 and 12/1/2021, 122 patients were initiated on HMA/VEN with 73 patients treated frontline (24 inpatient, 49 outpatient) and 49 treated in the relapsed/refractory setting (11 inpatient, 38 outpatient).
In the frontline setting, there was no difference in demographic data between those patients who initiated HMA/VEN inpatient versus outpatient while relapsed/refractory patients who were initiated inpatient were more likely to have a higher Charlson Comorbidity Index than that of those outpatients ( Table 1).
In terms of leukemia specific factors, frontline patients who received inpatient HMA/VEN were more likely to have a higher WBC at diagnosis. No differences in leukemia specific factors were seen amongst relapsed/refractory patients ( Table 1).
No difference in infections, TLS risk, or transfusion needs was seen amongst frontline patients who received HMA/VEN inpatient vs outpatient. Relapsed/refractory patients who received HMA/VEN inpatient had an increased risk of febrile neutropenia and required more pRBC and platelet transfusions than patients who received HMA/VEN outpatient. ( Table 1).
OS did not differ for frontline use of HMA/VEN amongst patients who initiated treatment inpatient versus outpatient [11 mo, (1-50) versus 6 mo (0-26), p 0.64]. In relapsed/refractory patients, those who were initiated on HMA/VEN outpatient had significantly higher OS (12.5 mo, range 0-76 mo) than those who were initiated on HMA/VEN inpatient (6 mo, range 2-50 mo, p 0.02) likely secondary to the increase in consolidative allo-SCT amongst outpatient relapsed/refractory HMA/VEN patients versus inpatient relapsed/refractory HMA/VEN patient (19 patients vs 1 patient, p 0.02).
Conclusion: Patients at our institution were able to be initiated on HMA/VEN as an outpatient for AML treatment without any perceived increase in TLS or infection risk in both the frontline and relapsed/refractory settings. Center and patient specific factors, such as travel distance and ability to administer supportive care, should be considered with outpatient administration of HMA/VEN.
Disclosures
Reagan:Pfizer: Research Funding; Rigel: Membership on an entity's Board of Directors or advisory committees.