Purpose Follicular lymphoma (FL) is a clinically and molecularly heterogeneous disease, watch and wait (W&W) remains a management therapeutic option in patients with advanced-stage, low-tumor-burden and asymptomatic FL in the rituximab era. We continue to use Groupe d'Etudes des Lymphomes Folliculaires criteria for active disease to initiate therapy. We sought to understand whether time to lymphoma treatment (TLT) after diagnosis in patients who managed by W&W was a factor affecting survival outcomes in FL.
Patients and Methods Between 2008 and 2022, 411 FL patients from 16 institutions in China were managed by W&W strategy, and their TLT was retrospectively evaluated. Patients were further divided into training and validation Cohorts. Logistic regression was used to identify and incorporate independent predictors of early TLT into a model with variable scoring. Model performance was evaluated through the area under the receiver operating characteristic curve (AUC) and goodness-of-fit statistics.
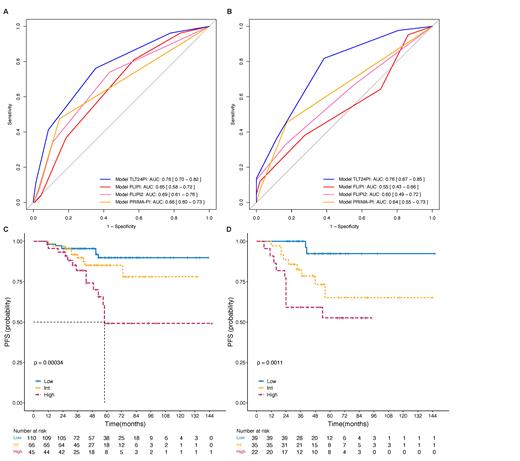
Results After a median follow-up of 46 months, 35 percent of W&W patients experience TLT within 24 months (TLT24) after diagnosis, and the 5-year progression free survival (PFS) rate was significantly lower than that of patients who were treatment-free at 24 months (62.3% vs. 89.5%). In multivariable analysis, five clinical factors were identified as independent predictors of TLT24: stage III-IV, β 2 microglobulin ≥ 3mg/L, lymphocyte-to-monocyte ratio<3.8, bone marrow involved and spleen enlargement. We calculated risk scores (TLT24PI) for each patient and defined three risk groups: low (0-1 points), intermediate (2 points), or high (3-5 points). Its AUC for TLT24 was 0.761 (95% CI, 0.698 - 0.823) in the development cohort and 0.761 (95% CI, 0.698 - 0.823) in the validation cohort. Risk groups were also associated with PFS ( P<0.001).
Conclusion In patients with FL who initially managed by W&W, TLT within 24 months after diagnosis was associated with poor outcomes. We developed a multivariable model that incorporates clinical and laboratory factors to identify patients at high risk for TLT24, which may be useful to identify candidates for early interventional treatment.
Disclosures
No relevant conflicts of interest to declare.