Human leukocyte antigen (HLA) well matched donor (related or unrelated) has remained the preferred donor source for patients undergoing HCT for hematological disease, but the probability of finding a suitable related or unrelated donor has varied based on ethnic groups. HLA-mismatch unrelated donor (MMUD) transplantation has improved access to HCT in underrepresented minority groups and patients of mixed race. Historically, MMUD HCT has been associated with inferior survival outcomes compared to matched unrelated donor HCT when using conventional platforms of GVHD prophylaxis. (PMID: 31449184, 20538804). Efforts to improve outcomes have investigated the addition of a third agent such as anti-thymocyte globulin (ATG), mini-methotrexate (MTX) or mycophenolate mofetil (MMF). More recently, a study by the National Marrow Donor Program (NMDP) demonstrated the feasibility of post-transplant cyclophosphamide (PTCy) based GVHD prophylaxis in MMUD HCT using bone marrow (BM) grafts (PMID: 33905264). Additionally, our group demonstrated promising overall survival (OS) and GVHD-free/relapse-free-survival (GRFS) rates in peripheral blood stem cell (PBSC) MMUD HCT using PTCy based GVHD prophylaxis (PMID: 34156440). Here, we compare outcomes of PBSC MMUD HCT among patients receiving GVHD prophylaxis with either: (1) tacrolimus plus sirolimus (T/S), (2) T/S plus mini-methotrexate (T/S/MTX), or (3) PTCy, MMF, and tacrolimus or sirolimus (PTCy).
Patients with various hematologic malignancies that underwent PBSC MMUD HCT (≥5/8 HLA-matched donors) at City of Hope between January 2005 and December 2021 were included in this retrospective review. Of 390 subjects included, GVHD prophylaxis included T/S (n=114), T/S/MTX (n=160), and PTCy (n=116). The median age was 51 (range:15-79), 53.3% of patients belonged to a racial/ethnic minority, and 34.8% (n=136) patients had disease risk index high or very high. Degree of HLA mismatch was 7/8 (91.3%), 6/8 (8.5%), and 5/8 (0.3%). Hematologic diseases included AML (40.3%), ALL (20.3%), MDS/MPN (15.9%), Lymphoma (16.9%), and CML/CMMol (6.7%). Most common conditioning regimens were fTBI-based (34.4%) and melphalan-based (57.2%). HCT comorbidity index was ≥ 3 in 30.8% (n=120), which was most common in PTCy compared to T/S and T/S/MTX: 48.3% vs. 23.7% vs. 23.1%, P<.001.
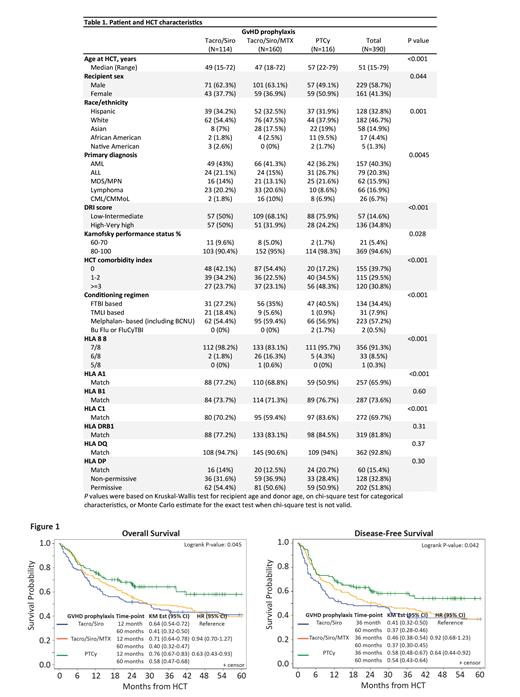
The rate of neutrophil engraftment (15 days vs. 15 days vs 16 days, P=0.002) and platelet engraftment (15 days vs. 16 days vs 27 days, P=0.004) were shorter in T/S compared to T/S/MTX and PTCy, respectively. With median follow-up of 5.4 years (range: 1.0-16.7) the 5-year OS was superior in PTCy (P=0.045) compared to T/S and T/S/MTX: 58.0% (95% CI: 46.6-67.8) vs. 41.1% (95% CI: 31.9-50.1) and 39.6% (95% CI: 32-47.2). The 5-year disease-free survival (DFS) was superior in PTCy (P=0.042) compared to T/S and T/S/MTX: 54.2% (95% CI: 43.3-63.9) vs. 37.1% (95% CI: 28.2-46.0) vs. 37.2% (95% CI: 29.7-44.6), respectively.
There was no significant difference in 5-year cumulative incidence of relapse (CIR) in T/S vs. T/S/MTX vs. T/S vs. PTCy: 29.1% (95% CI: 21.0-37.6) vs. 29.5% (95% CI: 22.6-36.7) vs. 22.1% (95% CI: 14.3-31.0), respectively. Additionally, there was no significant difference in 2-year NRM between the cohorts (P=0.459): 24.6% (95% CI: 17.1-32.9) vs. 23.1% (95% CI: 16.9-29.9) vs. 20.4% (95% CI: 13.5-28.4), respectively. The rate of 100-day Grade III-IV acute GVHD were similar between in the cohorts (P=0.321): 19.3% (95% CI: 12.6-27.0) vs. 18.1% (95% CI: 12.6 -24.5) vs. 12.9% (95% CI: 7.6-19.7). When comparing T/S vs. T/S/MTX and PTCy, the incidence of chronic GVHD was less in the PTCy cohort at both 1-year and 2-years (P<.001) with 1-year incidence of 55.0% (95% CI: 45.1-63.7) vs. 61.9% (95% CI: 53.8-68.9) vs. 38.9% (95% CI: 30-47.8), respectively, and 2-year incidence of 58.9% (95% CI: 49.0-67.6) vs. 66.2% (95% CI: 58.3-73.1) vs. 42.7% (95% CI: 33.4-51.6), respectively.
To our knowledge, this is the largest reported single center outcome of PBSC MMUD HCT. In this study, we demonstrate the superiority of PTCy based GVHD prophylaxis compared to T/S and T/S/MTX in outcomes of 5-year OS (P=0.045), 5-year DFS (P=0.042), and incidence of chronic GVHD (P<.001). Our experience further supports the superiority of PTCy as the preferred GVHD prophylaxis in the PBSC MMUD HCT setting achieved by lowering chronic GVHD and associated complications.
Disclosures
Koller:treadwell therapuetics: Consultancy, Other: safety review committee; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; takeda: Consultancy, Speakers Bureau. Sandhu:Autolus Therapeutics: Consultancy; City of Hope Medical Center: Current Employment. Aldoss:KiTE: Consultancy; Pfizer: Consultancy; Sobi: Consultancy; Jazz: Consultancy; Amgen: Consultancy, Honoraria; Takeda: Consultancy. Ali:Blueprints: Speakers Bureau; Pharmaessentia: Consultancy; GSK: Consultancy; Karyopharm: Consultancy; Incyte: Research Funding; BMS: Speakers Bureau. Salhotra:Jazz Pharma: Research Funding; OrcaBio: Research Funding; Sanofi: Speakers Bureau; Sobi: Membership on an entity's Board of Directors or advisory committees; Rigel Pharma: Research Funding; Kura Oncology: Research Funding; Gilead: Research Funding; BMS: Research Funding. Aribi:Kite, a Gilead Company: Consultancy; Seagen: Consultancy. Artz:Abbvie: Consultancy; Magenta Therapeutics: Other: Advisory Board; Astra Zeneca: Other: Advisory Board; Radiology Partner: Current equity holder in private company, Other: Spouse equity interest. Becker:Accordant Health Services: Membership on an entity's Board of Directors or advisory committees; Glycomimetics: Research Funding; Pfizer: Research Funding; GPCR Therapeutics: Research Funding. Pullarkat:Pfizer: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Servier: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau. Stein:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Marcucci:Ostentus Therapeutics: Current equity holder in private company, Research Funding. Nakamura:BMT CTN Steering Committee: Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Other: grant reviewer; Napajen: Consultancy; Blue Bird: Consultancy; NCTN Lymphoma Steering Committee: Membership on an entity's Board of Directors or advisory committees; Omeros: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: research collaboration; Mt. Sinai: Other: Acute GVHD; International Consortium: Other: consortium chair; Miyarisan: Research Funding; NCCN: Other: guideline panel for HCT; Sanofi: Consultancy. Al Malki:Tscan: Consultancy.