Introduction
Since 2017, 6 chimeric antigen receptor T-cell (CAR-T) therapies have been approved for Acute Lymphoblastic Leukemia (ALL), Mantle Cell Lymphoma (MCL), Diffuse Large B-cell Lymphoma (DLBL), Follicular Lymphoma (FL), and Multiple Myeloma (MM) and have changed the treatment landscape immensely. There is significant demographic and geographic under-representation in pivotal clinical trials leading to drug approvals for leukemias, lymphomas and MM compared with the population affected. We investigated whether similar disparities exist in pivotal CAR-T trials.
Method
Clinical Trials leading to CAR-T approval were identified from the US Food and Drug Administration databases. Demographic data was collected from clinicaltrials.gov and primary manuscripts. Standard descriptive statistics were used to analyze the data with stratification by race, ethnicity, sex, and malignancy subtypes. Total incidence of respective malignancies and the demographic representation was obtained from SEER data.
Results
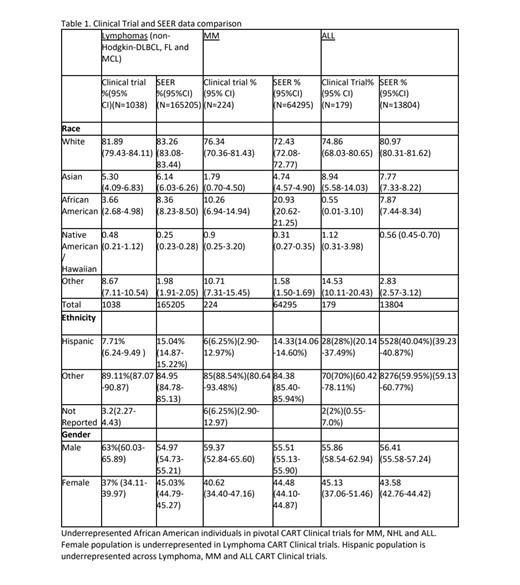
A total of 10 out of 11 (90.91%) clinical trials leading to CAR-T approval reported data on race and 8 (72.72%) on ethnicity. These trials led to approvals for MM, non-Hodgkin lymphoma (DLBCL, FL and MCL), and ALL. These trials included 1441 patients of which 1155 (80.15%) were reported as White. NCT02445248, for Tisagenlecleucel (tisa-cel) lacked racial data and was excluded from analysis. Data are summarized in Table 1.
For MM, 2 CARTs have been approved, Idecabtagene vicleucel and Ciltacabtagene autoleucel (cilta-cel). African American (AA) representation in these pivotal trials, NCT03361748 and NCT03548207 (CARTITUDE-1), respectively was 4.7 % and 17.5 %. In contrast, SEER data shows that AA population represent 21% of the incident MM cases over the last 5 years (2016-2021).
In ALL, 2 CARTs, tisa-cel and Brexucabtagene Autoleucel (brexu-cel) have been approved. AAs had 0.55% of representation in the CAR-T ALL clinical trials, whereas they represent 7.5% of all ALL cases in the past 5 years. For follicular lymphoma, Tisa-cel and Axicabtagene ciloleucel (Axi-cel) have been approved as third line treatment. For DLBCL, lisocabtagene maraleucel (liso-cel), tisa-cel and axi-cel have been approved as third line with liso-cel and axi-cel also having second line DLBCL indication. Brexu-cel has been approved for third line MCL. Under-representation of AAs with respect to disease distribution was noted in all these trials. Female patients were also disproportionately under-represented in lymphoma trials overall. Ethnicity was reported less frequently in aforementioned studies but available data suggests Hispanic groups being underrepresented in these trials.
Conclusion
There are significant demographic under-representations and imbalances in pivotal clinical trials leading to CART approval compared with the population affected. Efforts need to be directed at ensuring racial and ethnic inclusivity and equity in trials for better applicability to the “real world”. Trials should also consistently report demographic data, especially ethnic and racial, for better transparency and accurate analysis of presented data.
Disclosures
Kota:Kite: Honoraria; Novartis: Honoraria; Incyte: Research Funding; Pfizer: Honoraria. Keruakous:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees. Cortes:Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Research Funding; Forma Therapuetic: Consultancy; Gilead: Consultancy; Takeda: Consultancy, Honoraria; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding.