Introduction:
Hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone) is a commonly used regimen in acute lymphoblastic leukemia (ALL) for a total of 4 A/B intensive courses. This regimen is often limited due to multiple complications including organ dysfunction and medication toxicities along with high rates of relapse or disease progression. Prior studies have indicated five-year overall survival (OS) of 39% with severe prolonged myelosuppression being a common complication. In one study 6% of patients died during induction therapy. Here we provide clinical outcomes of non-transplanted patients with ALL receiving hyper-CVAD regimen in our tertiary referral center. We assessed overall survival, mortality rates, and complications during induction therapy with hyper-CVAD in our cohort of adult patients.
Methods:
We conducted a comprehensive, retrospective study at our center, evaluating outcomes of non-transplant eligible ALL patients treated from February 2003 to October 2022. We excluded patients without all available diagnostic information, and those who underwent allogeneic stem cell transplantation (SCT). We classified the patient based on their BCR - ABL1 gene status. Groups were subcategorized by highest cycle of Hyper-CVAD completion, with reason for cessation (medication toxicity/organ dysfunction, lost to follow up, progressive disease/relapse) elicited for those who did not complete all treatment cycles. Overall survival was analyzed using T-Test, ANOVA and Bartlett's test. Statistics are descriptive and our institutional review board approved this retrospective study.
Results:
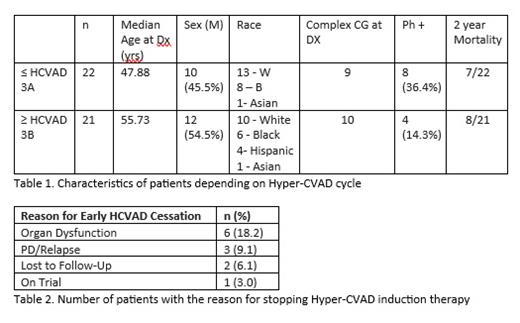
We report outcomes of 43 ALL patients who did not undergo SCT. Median age at diagnosis was 52 years (range of 18-71 years). 33/43 (76.7%) patients received Hyper-CVAD regimen. 2/33 (6.1%) completed the entire course through 4B. 12 (36.3%) out of 33 patients received less than cycle 3b; 21/33 (63.6%) received at least cycle 3b. 2/6 (33.3%) completed up to cycle 1B, 2/11 (18.2%) completed up to cycle 2B, 13/25 (52%) completed up to cycle 3B, and 2/33 (6.1%) completed up to cycle 4B. 12/33 (36.4%) of patients were not able to complete more than cycle 3A due to organ dysfunction and intolerance, being lost to follow up, progressive disease or relapse, or participation in a trial. For the patients who received less than cycle 3b, the reasons for early discontinuation were: organ dysfunction 6/12 (50%), progressive disease or relapse 3/12 (25%), lost to follow up 2/12 (16.7%), or participation in a trial 1/12 (8.3%).
Median overall survival (OS) for patients who received up to cycle 3A was 640 days compared to a median of 711 days for those who received at least cycle 3B. Median OS for patients who received up to cycle 3A in the Ph-negative group was 21.0 months and 23.1 months in the Ph-positive group. Median OS for patients who received at least 3B in the Ph-negative group was 20.9 months and 23.9 months in the Ph-positive group.
Conclusion:
Hyper-CVAD is the mainstay of ALL treatment. Induction chemotherapy in ALL is commonly associated with myelosuppression and documented infections (sepsis, pneumonia, port infection). Other significant side effects include neurotoxicity, liver and kidney failure. Induction chemotherapy in some patients was a limiting factor in completing therapy. We report the different reasons for terminating the treatment course to be: organ dysfunction or intolerance such as liver injury, neutropenic fever, septicemia, subarachnoid hemorrhage, renal failure, congestive heart failure, respiratory failure requiring intubation, port infection, cytokine release syndrome, and neurotoxicity.
Median OS was longer in the Ph positive subgroup but the mortality rates for this subtype was not different between the patients who received up to cycle 3A verses those that received at least cycle 3B. In this study, our descriptive analysis did not show a clinically meaningful difference in survival in patients who did not finish the total induction course.
A limitation of this study was not analyzing intrathecal prophylaxis as involvement of the central nervous system is a major clinical concern.
Herein, we suggest a personalized approach in treating ALL patients considering infection risk, disease cytogenetic risk and depth of response to help adjusting the number of induction cycles based on the mentioned risk factors.
Disclosures
Keruakous:BMS: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cortes:Novartis: Consultancy, Research Funding; Forma Therapuetic: Consultancy; Abbvie: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy; Takeda: Consultancy, Honoraria; Pfizer: Consultancy, Research Funding. Kota:Novartis: Honoraria; Kite: Honoraria; Pfizer: Honoraria; Incyte: Research Funding.