Introduction:
The Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) are characterized by the clonal proliferation of differentiated hematologic cells. However, there is a limited understanding of how perturbations at the transcriptional level may contribute to accelerated or blast-phase disease, together referred to here as transformation. Here we characterize transcriptomic characteristics and cell type composition of MPNs along the transition to transformed disease.
Methods:
RNAseq and clinical data were collected on 261 patients who were part of BeatAML, including those with MPN, de-novo AML, and transformed MPN. This was combined with data from 56 transformed MPN patients seen at MSK, 11 of whom also had paired chronic-phase data available. Differential expression analysis was performed with DESeq2; both GSEA and GSVA were used to evaluate gene-set enrichment and pathways of biological relevance. A custom RNA-seq lineage deconvolution algorithm was used to estimate hematopoietic stage proportions and was used for unsupervised hierarchical clustering of cases. Kaplan-Meier methods were used to estimate survival among transformed MPN lineage subtypes.
Results:
The initial analysis included 207 de novo AML cases and 75 MPN-BP cases from BeatAML and MSK on which RNAseq and molecular data were integrated across cohorts. Unsupervised analysis was performed using hierarchical clustering applied to batch corrected, variance stabilized RNA-seq count data. Transformed MPN cases were enriched in 2 of 6 clusters (N = 10, 33.3%; N = 36, 54.5%). Notably, the molecular characteristics in these clusters are varied, suggesting that multiple genomic perturbations may lead to similar pathways of transformation.
Gene set variation analysis (GSVA) demonstrated a coalescence of transcriptomic perturbation into specific pathways for transformed MPNs, such as hematopoietic stem cell gene sets (q-value = 0.00018). On the contrary, AML cases typically demonstrated upregulation in later stages of hematopoiesis, such as monocytes (q-value =0.00016). In evaluating specific genes differentially expressed between transformed MPNs compared to de novo AML and tAML patients, microRNA MIR29B1 was particularly upregulated in MPN-BP (logFC = 9.52, q < 0.01), while the proto-oncogene CT45A1 had decreased expression in transformed MPN compared to other AMLs (logFC = -7.47, q < 0.01).
Paired GSEA of the 11 patients with myelofibrosis MF and transformed disease showed that transformed disease was enriched for CD34-positive HSCs (NES = 2.64, q-value < 0.0001) and E2F transcription factors (NES 2.66, q-value < 0.0001), as well as doxorubicin resistance (NES 2.40, q-value < 0.0001).
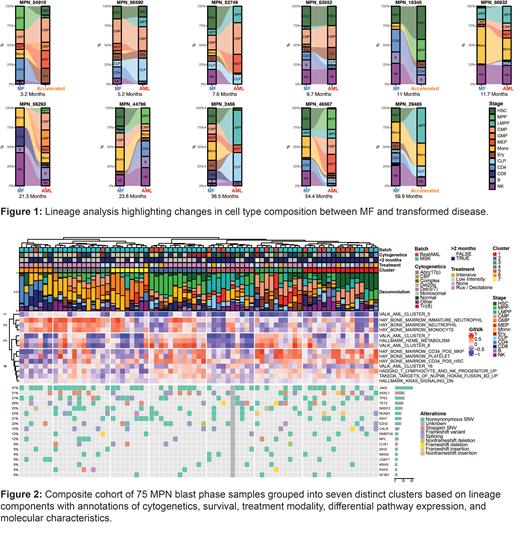
Given enrichment of the CD34 positive HSC geneset, we performed our RNA-seq based lineage deconvolution on each of the paired samples ( Figure 1). MPNs appeared to transform in a heterogenous fashion reminiscent of the biological diversity in AML. In aggregate, transformation was associated with decreased monocytic (p = 0.002), natural killer (NK) (p = 0.042) character and expansion of common lymphoid progenitor character (p = 0.022). Lineage composition changes varied, with certain samples showing HSC expansion (ID: 54910, ID: 46567) and others exhibiting LMPP expansion (ID: 29485, 2456).
Figure 2 defines seven distinct clusters of transformed MPN patients, driven by their lineage components. While these clusters are transcriptionally distinct, we again see that they are not defined by their genomic or cytogenetic alterations. We evaluated for a difference in overall survival (OS) between each cluster and intermediate-risk de novo AML. We found significantly worse OS among patients in three MPN-BP clusters - MPP (p =0.008), LMPP (p = 0.038), and GMP (p < 0.001) - represented as clusters 2, 5, and 7 in Figure 2. Compared to intermediate risk AML, transformed MPNs had significantly worse OS (HR: 1.79 95% CI: 1.18, 2.71, p = 0.0057).
Discussion:
To our knowledge, this is the first ever description of the transcriptional characteristics of MPN-BP, its comparison to de novo AML, and the first paired comparison of transcriptional and lineage characteristics for patients with chronic-phase MPNs that subsequently transformed. Our study provides valuable insights into the transcriptomic landscape of transformed MPN including both accelerated and blast phase disease, and draws novel contrasts to de novo AML.
Disclosures
Menghrajani:Gilead: Consultancy. Rampal:Incyte: Consultancy; Dainippon: Consultancy; Sumitomo: Consultancy; Kartos: Consultancy; Celgene-BMS: Consultancy; Ryvu: Research Funding; Stemline: Research Funding; GSK-Sierra: Consultancy; Constellation: Research Funding; CTI BioPharma Corp: Consultancy; Galecto: Consultancy; Pharmaessentia: Consultancy; Morphosys/Constellation: Consultancy; Servier: Consultancy; Zentalis: Consultancy; Karyopharm: Consultancy; Incyte: Research Funding; Zentalis: Research Funding. Levine:Lilly: Honoraria; Mission Bio: Membership on an entity's Board of Directors or advisory committees; Ajax: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria; Roche: Honoraria; Novartis: Consultancy; AstraZeneca: Consultancy, Honoraria; Janssen: Consultancy; Qiagen: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Isoplexis: Membership on an entity's Board of Directors or advisory committees; C4 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Prelude: Membership on an entity's Board of Directors or advisory committees; Auron: Membership on an entity's Board of Directors or advisory committees; Zentalis: Membership on an entity's Board of Directors or advisory committees, Research Funding.